An activated near-infrared small molecule fluorescent probe and its preparation method and application
A fluorescent probe and small molecule technology, applied in the field of biological probes, can solve the problems of reducing the accuracy of imaging results, being easy to be cleared, and difficult to monitor the occurrence and progress of acute kidney injury, achieving high imaging detection and analysis, avoiding clearing, The effect of high signal-to-background ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
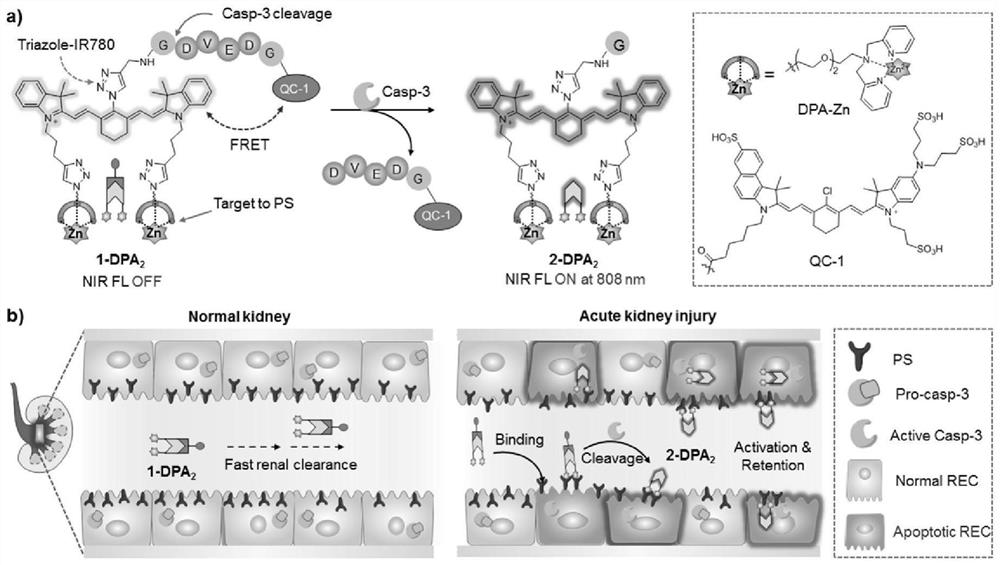
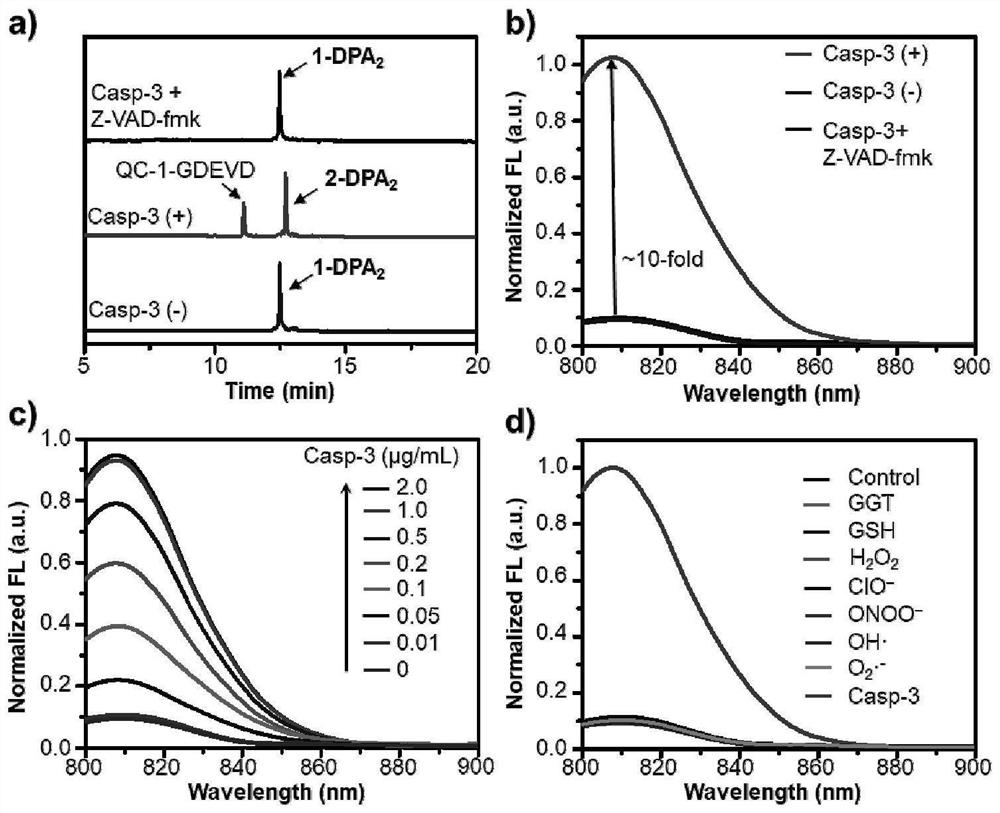
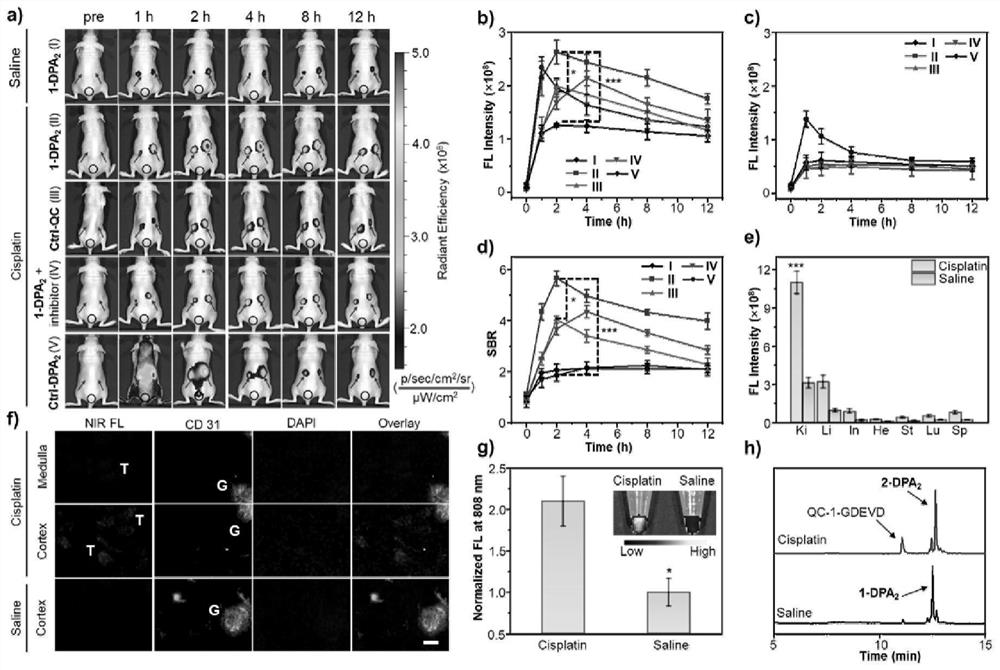
[0050] Probe 1-DPA 2 Design, synthesis and characterization
[0051] The synthetic route of 2-[2-(2-Azidoethoxy)ethoxy]-N,N-bis (2-pyridinylmethyl) ethanamine intermediate compound 3 (CAS: 1613538-61-6) is as follows:
[0052]
[0053] Reaction conditions: (a) NaN 3 ,H 2 O,90℃,24h,96%; (b) TsCl, triethylamine, DCM, r.t., 4h, 83%; (c) 2,2-Dimethylpyridinamine, triethylamine, CH 3 CN,80℃,24h,77%。
[0054] Synthesis of Compound 1: 2-Chloroethoxy-2-ethoxydiethanol (991 mg, 5.9 mmol), NaN 3 (462mg, 7.1mmol) dissolved in secondary water (5mL), stirred at 90 °C for 24 hours. After the reaction was completed, 20mL of ethyl acetate was added to the reaction mixture for extraction, a total of 3 times, and the organic layer was anhydrous Na 2 SO 4 Dry. After filtering to remove the precipitate, the solvent is removed by steaming to give compound 1 as a transparent colorless liquid. Yield: 955 mg (96%) 1 H NMR(400MHz,Chloroform-d)δ3.74(dd,J=8.1,3.8Hz,2H),3.69(d,J=2.8Hz,6H),3.62(dd,J=5.3,3.8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com