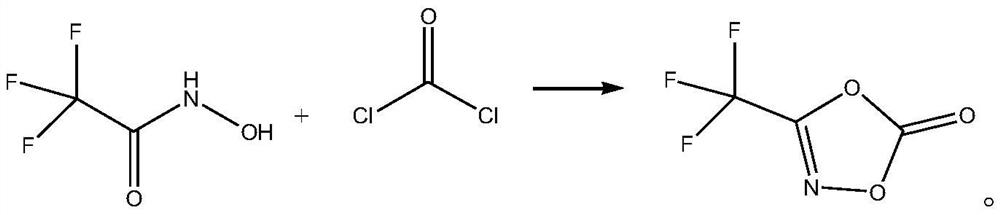
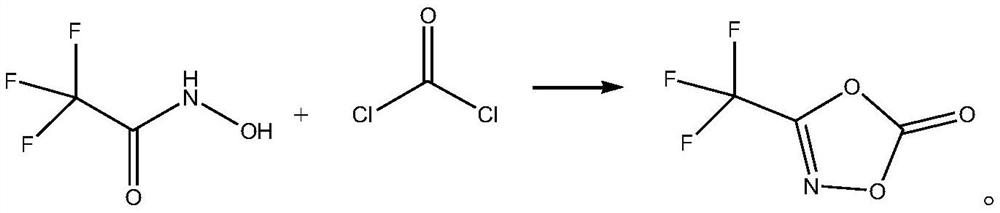
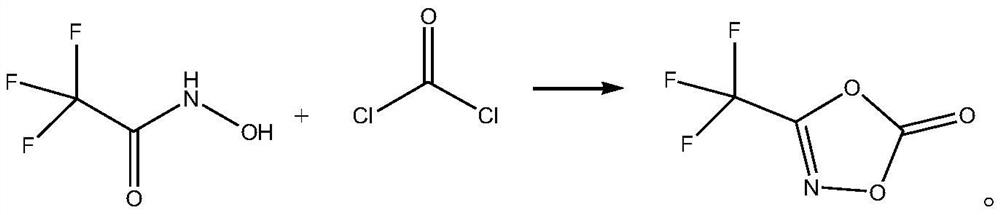
Synthesis method of 3-trifluoromethyl-1, 4, 2-dioxazole-5-one
A technology of trifluoromethyl and synthesis method, applied in the field of battery electrolyte additives, can solve the problems of imperfect manufacturing and purification methods, poor purity, low productivity, etc., and achieves mild reaction process, simple reaction process, and improved yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthetic method of 3-trifluoromethyl-1,4,2-dioxazol-5-one, its synthetic steps are as follows:
[0019] Dissolve 14.84 g of solid phosgene (0.15 mol) in 74 mL of toluene to obtain a toluene solution of phosgene;
[0020] Add 12.9g (0.1mol) of 2,2,2-trifluoro-N-hydroxyacetamide to 6.7mL of diethylene glycol dimethyl ether, stir to dissolve, then control the system temperature to 30°C, and maintain the system temperature at Slowly add the toluene solution of phosgene at 30°C, then slowly raise the temperature to 60°C, maintain 60°C, stir the reaction until the reaction of 2,2,2-trifluoro-N-hydroxyacetamide is complete, then raise the temperature to 110°C to evaporate the volatiles (solvent, etc.), and then fractionated by a Vigreux fractionating column to obtain 8.51g of 3-trifluoromethyl-1,4,2-dioxazol-5-one, with a yield of 54.89% and a purity of 99.6%. The specific chemical reaction formula as follows:
[0021]
Embodiment 2
[0023] The synthetic method of 3-trifluoromethyl-1,4,2-dioxazol-5-ketone, its synthetic steps are as follows
[0024] Dissolve 16.82g of solid phosgene (0.17mol) in 84mL of toluene to obtain a toluene solution of phosgene;
[0025] Add 12.9g (0.1mol) of 2,2,2-trifluoro-N-hydroxyacetamide to 6.9mL of diethylene glycol dimethyl ether, stir to dissolve, then control the system temperature to 25°C, and maintain the system temperature at Slowly add the toluene solution of phosgene at 25°C, then slowly raise the temperature to 70°C, maintain 70°C, stir the reaction until the reaction of 2,2,2-trifluoro-N-hydroxyacetamide is complete, then raise the temperature to 118°C to evaporate the volatiles (solvent, etc.), and then fractionated by a Vigreux fractionating column to obtain 8.72 g of 3-trifluoromethyl-1,4,2-dioxazol-5-one, with a yield of 56.25% and a purity of 99.5%.
Embodiment 3
[0027] The synthetic method of 3-trifluoromethyl-1,4,2-dioxazol-5-one, its synthetic steps are as follows:
[0028] Dissolve 13.85 g of solid phosgene (0.14 mol) in 84 mL of toluene to obtain a toluene solution of phosgene;
[0029] Add 12.9g (0.1mol) of 2,2,2-trifluoro-N-hydroxyacetamide to 6.5mL of diethylene glycol dimethyl ether, stir to dissolve, then control the system temperature to 20°C, and maintain the system temperature at Slowly add the toluene solution of phosgene at 20°C, then slowly raise the temperature to 65°C, maintain 65°C, stir the reaction until the reaction of 2,2,2-trifluoro-N-hydroxyacetamide is complete, then raise the temperature to 115°C to evaporate the volatiles (solvent, etc.), and then fractionated by a Vigreux fractionating column to obtain 8.43 g of 3-trifluoromethyl-1,4,2-dioxazol-5-one, with a yield of 54.38% and a purity of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com