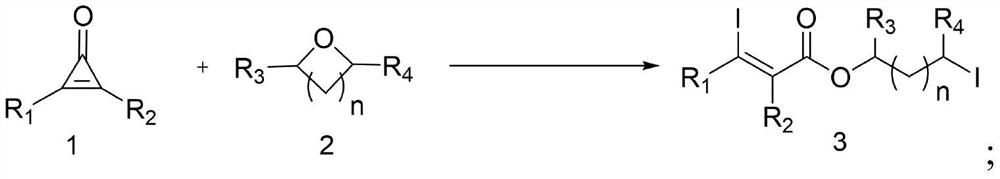
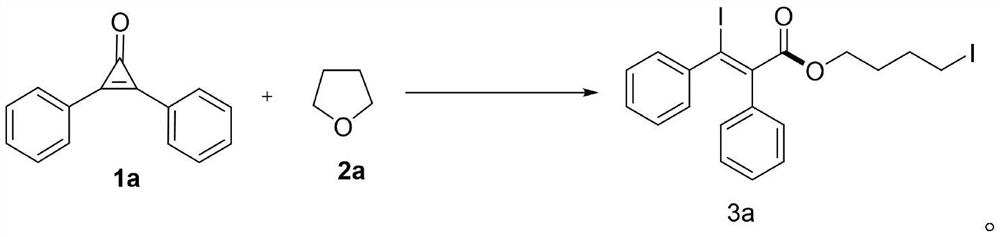
Ring-opening diiodination reaction method of cyclopropenone and oxygen heterocyclic compound
A cyclopropenone, iodination reaction technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problem of inability to prepare 3-iodoacrylate, etc., and achieve excellent substrate range, reaction Simple operation, high atomic economy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 22
[0035]
[0036] Cyclopropenone (0.2mmol, 1.0 equivalents) shown in formula 1b, I 2 (0.4mmol, 2.0 equivalents), tetrahydrofuran (0.3mmol, 1.5 equivalents) and CH 3 CN (2 mL) was loaded into a 10 mL reaction tube. The reaction mixture was stirred at 120°C for 10 hours. After completion of the reaction, 2.0 ml of water was added to the reaction mixture, the reaction solution was extracted with ethyl acetate (3 x 2 mL), the combined organic layers were dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The remaining residue was purified by silica gel column chromatography to obtain the 3-iodoacrylate compound represented by formula 3b with a yield of 50%. Structure Characterization: 1 H NMR (500MHz, Chloroform-d) δ7.19–7.11(m, 6H), 6.98(d, J=8.4Hz, 2H), 4.30(t, J=6.2Hz, 2H), 3.19(t, J= 6.8Hz,2H),1.99–1.93(m,2H),1.90–1.83(m,2H),1.25(s,9H),1.22(s,9H). 13 C NMR (125MHz, Chloroform-d) δ169.0, 151.4, 150.9, 143.1, 139.1, 132.2, 129.1, 128....
Embodiment 23
[0038]
[0039] Cyclopropenone (0.2mmol, 1.0 equivalents) shown in formula 1c, I 2 (0.4mmol, 2.0 equivalents), tetrahydrofuran (0.3mmol, 1.5 equivalents) and CH 3 CN (2 mL) was loaded into a 10 mL reaction tube. The reaction mixture was stirred at 120°C for 10 hours. After completion of the reaction, 2.0 ml of water was added to the reaction mixture, the reaction solution was extracted with ethyl acetate (3 x 2 mL), the combined organic layers were dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The remaining residue was purified by silica gel column chromatography to obtain the 3-iodoacrylate compound represented by formula 3c with a yield of 56%. Structure Characterization: 1 H NMR (500MHz, Chloroform-d) δ7.18–7.14(m,2H),7.07–7.03(m,2H),6.89-6.83(m,4H),4.30(t,J=6.2Hz,2H), 3.20(t,J=6.8Hz,2H),1.97–1.91(m,2H),1.89–1.82(m,2H). 13 C NMR (125MHz, Chloroform-d) δ168.3, 162.2(J=247.8Hz), 162.2(J=248.5Hz), 142.9, 137.9(J=3.7Hz), 130.9...
Embodiment 24
[0041]
[0042] Cyclopropenone (0.2mmol, 1.0 equivalents) shown in formula 1d, I 2 (0.4mmol, 2.0 equivalents), tetrahydrofuran (0.3mmol, 1.5 equivalents) and CH 3 CN (2 mL) was loaded into a 10 mL reaction tube. The reaction mixture was stirred at 120°C for 10 hours. After completion of the reaction, 2.0 ml of water was added to the reaction mixture, the reaction solution was extracted with ethyl acetate (3 x 2 mL), the combined organic layers were dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The remaining residue was purified by silica gel column chromatography to obtain the 3-iodoacrylate compound represented by formula 3d with a yield of 50%. Structure Characterization: 1 H NMR (500MHz, Chloroform-d) δ7.18–7.09(m,6H),7.02–7.00(m,2H),4.31–4.28(m,2H),3.19(t,J=6.6Hz,2H), 1.93(m,2H),1.84(m,2H). 13 C NMR (125MHz, Chloroform-d) δ168.1, 143.0, 140.2, 134.5, 134.4, 133.3, 130.6, 130.0, 128.7, 128.5, 99.5, 64.8, 29.9, 29.3, 5.3. H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com