CD74-ROS1 rearrangement DNA (deoxyribonucleic acid) standard substance for molecular diagnosis, RNA (ribonucleic acid) standard substance for molecular diagnosis and application of CD74-ROS1 rearrangement DNA standard substance and RNA standard substance
A technology of molecular diagnosis and standard products, which is applied in the direction of DNA/RNA fragments, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problems of low success rate, small amount, non-reproducible samples, etc., and achieve complete and accurate breakpoints Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides a method for preparing cells for DNA standard or RNA standard, comprising the steps of:
[0041] (1) Construct the sgRNA targeting intron 6 of the CD74 gene on the vector pX330 to obtain plasmid C4;
[0042] (2) Construct the sgRNA targeting intron 33 of the ROS1 gene on the vector pX330 to obtain plasmid R3;
[0043] (3) Co-transfect the host cells with plasmid C4 and plasmid R3 to obtain recombinant cells;
[0044] (4) Perform monoclonalization of recombinant cells to obtain cells for preparing DNA standards or RNA standards.
[0045] In the present invention, the sequence of the sgRNA targeting intron 6 of the CD74 gene in step (1) is preferably as shown in SEQ ID NO.18.
[0046] In the present invention, the sequence of the sgRNA targeting intron 33 of the ROS1 gene in step (2) is preferably as shown in SEQ ID NO.27.
[0047] In the present invention, the monoclonalization in step (4) is preferably carried out by the limiting d...
experiment example 1
[0056] Select mother cells with higher plasmid transfection efficiency as alternative infected host cells, as shown in Table 2:
[0057] Table 2 Sources and media of different host cell lines
[0058] cell line source culture medium HEK293 ATCC;CRL-1573 DMEM+10%FBS HCT116 ATCC;CCL-247 McCoy's 5a+10%FBS DLD-1 ATCC;CRL-2577 RPMI-1640+10%FBS RKO ATCC;CCL-221 MEM+10%FBS SW48 ATCC;CCL-231 DMEM+10%FBS
[0059] According to the instructions published by ATCC, culture 5 cell lines, adjust to the exponential growth phase, plate the cells into 6-well plates at a density of 1x10e6cell / well, and culture overnight;
[0060] The next day, the cells were observed under a microscope. When the confluence of the cells reached 80%, the plasmid PX458 with GFP was transfected into 5 kinds of host cells, 1.5 μg / well, and the transfection method followed the instructions of lipo3000. The expression of GFP in the cells was observed with ...
experiment example 2
[0093] ddPCR detects the copy number of CD74-ROS1 rearrangement standard cells at the DNA level:
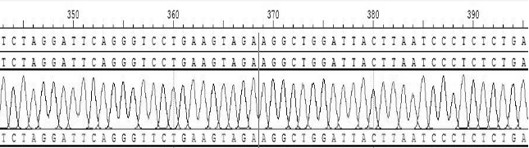
[0094] Clone No. 23 with CD74-ROS1 rearrangement was selected, and ddPCR was designed to detect the frequency of the rearrangement of interest, and the target sequence was shown in SEQID NO.35.
[0095] Design ddPCR for the sequence of interest:
[0096] CD74-ROS1-23-F is shown in SEQ ID NO.36;
[0097] CD74-ROS1-23-R is shown in SEQ ID NO.37;
[0098] CD74-ROS1-23-P is shown in SEQ ID NO.38;
[0099] ROS1-T-23-F is shown in SEQ ID NO.39;
[0100] ROS1-T-23-R is shown in SEQ ID NO.40;
[0101] ROS1-T-23-P is shown in SEQ ID NO.41.
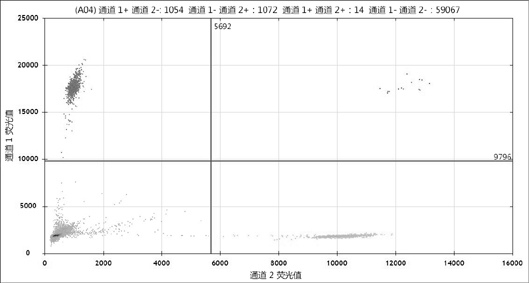
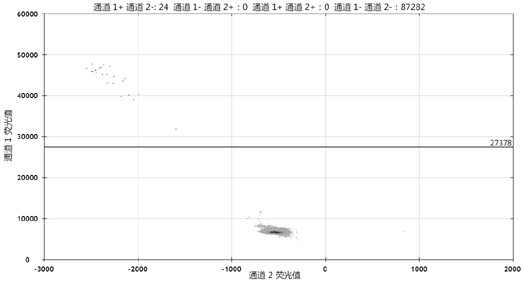
[0102] The ddPCR test showed that the rearrangement frequency of CD74-ROS1 in clone 23 was 50%, that is, there were 2 copies of ROS1 in HCT116, 1 copy was rearranged, and the other 1 copy did not occur. Such as Figure 4 shown. The FAM probe detects the copy number of CD74-ROS1 rearrangement, and the VIC probe detects the overall copy number o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com