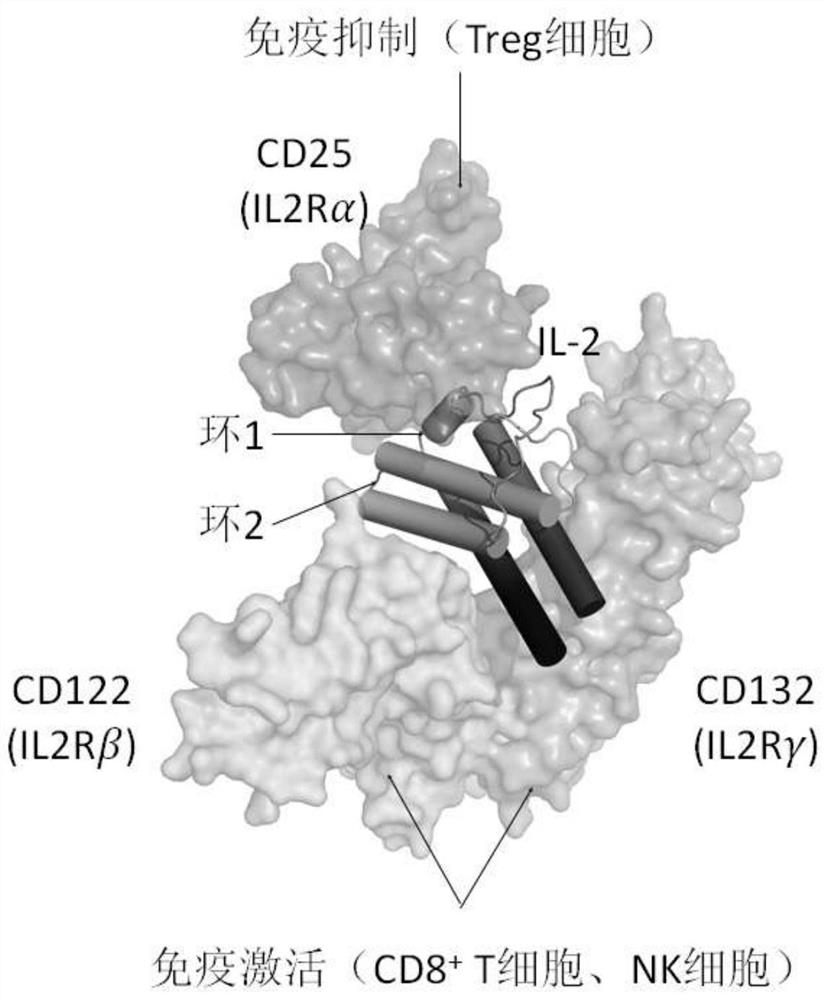
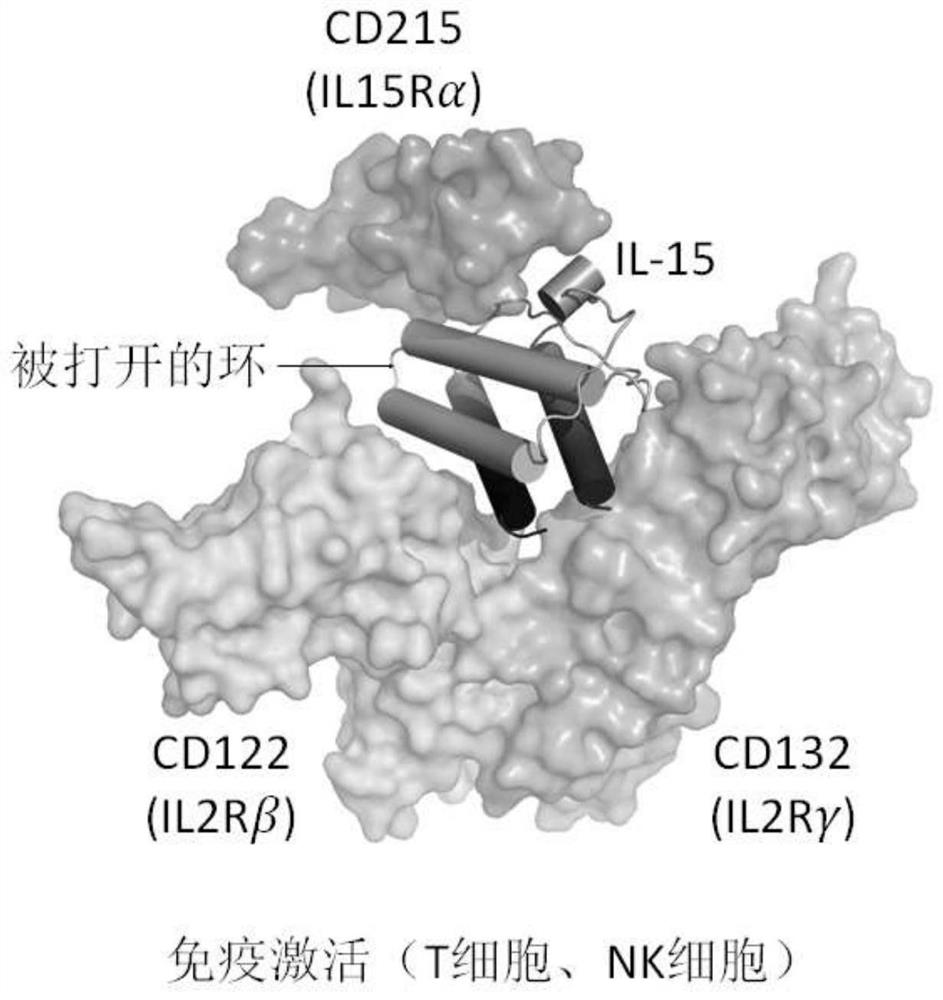
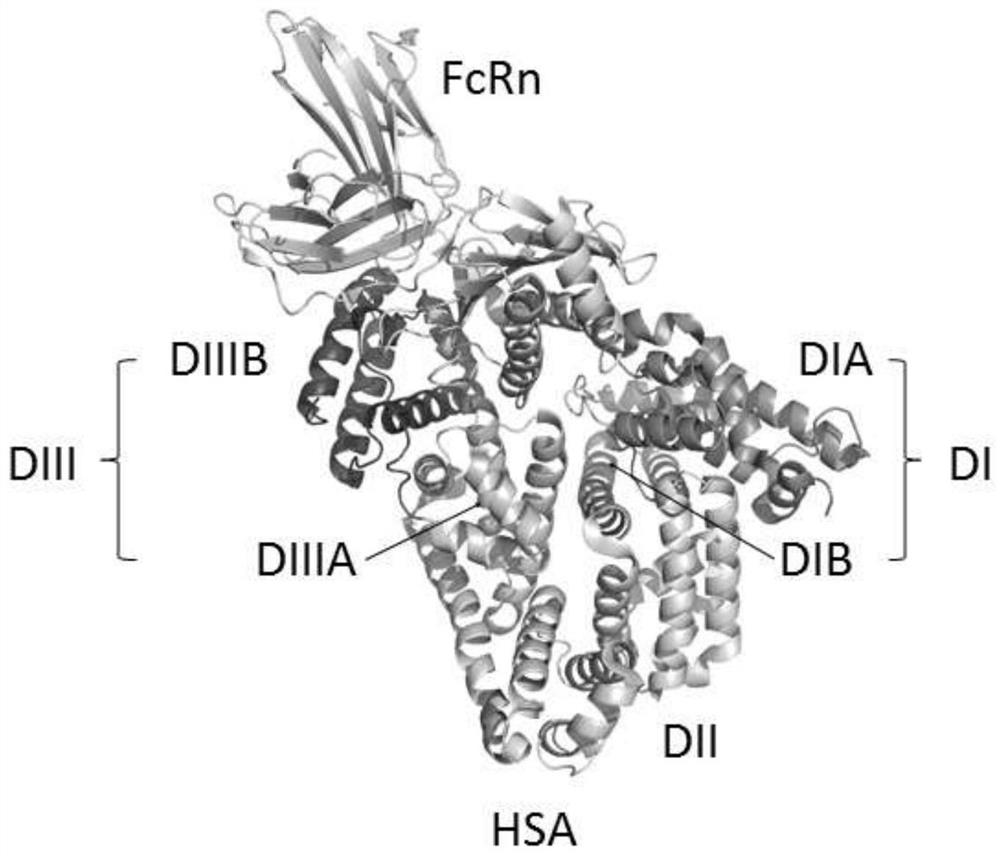
Fusion polypeptides and uses thereof
A technology for fusing peptides and binding sites, which is applied in the direction of hybrid peptides, plasma life extension fusion, peptides, etc., can solve problems such as short half-life in vivo and safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0095] HSA can also be modified to improve its properties. For example, the free cysteine in wild-type HSA can be substituted for other amino acids, such as serine. In some embodiments, the HSA comprises the amino acid substitution C58S.
[0096] In some embodiments, the carrier protein has at least 70%, 80%, 90%, 95%, 96%, 97%, 98%, or 99% sequence identity to SEQ ID NO: 16, and has The same or similar structure as type HSA.
[0097] In some embodiments, the N-terminus and C-terminus of the polypeptide of interest are linked to the carrier protein via a linker. In some embodiments, the N-terminus of the polypeptide of interest is connected to the carrier protein through a linker, and the C-terminus of the polypeptide of interest is directly connected to the carrier protein. In some embodiments, the N-terminus of the polypeptide of interest is directly connected to the carrier protein, and the C-terminus of the polypeptide of interest is connected to the carrier protein t...
Embodiment 1
[0138] Embodiment 1, the construction of expression vector
[0139] The construction of the expression vector was completed by Nanjing GenScript Biotechnology Co., Ltd., including: synthesizing the nucleic acid encoding the amino acid sequence of SEQ ID NO: 12-15, the nucleic acid additionally comprising the nucleotide sequence encoding the signal peptide of SEQ ID NO: 18 ; Construction of the nucleic acid into the mammalian cell expression vector pcDNA3.4 by molecular cloning methods, and amplification and purification of the plasmid, etc.
Embodiment 2
[0140] Embodiment 2, the expression of fusion polypeptide
[0141] In this example, 293-6E cells were transfected with the nucleic acid encoding the fusion polypeptide for eukaryotic expression. The expression and purification of the fusion polypeptide were completed by Nanjing GenScript Biotechnology Co., Ltd. The process is briefly described as follows:
[0142] -293-6E cells in serum-free FreeStyle TM 293Expression medium (Thermo Fisher Scientific, Carlsbad, CA, USA), placed in Erlenmeyer flasks (Corning Inc., Acton, MA), cultured on a shaking incubator (VWR Scientific, Chester, PA), culture conditions at 37°C with 5% CO 2 .
[0143] - The day before transfection, dilute the cells to an appropriate density.
[0144] - On the day of transfection, the plasmid mixture (the mass ratio of the plasmid encoding the first polypeptide and the second polypeptide is 1:1) and the transfection reagent (such as polyetherimide, Polyetherimide, PEI) in an appropriate ratio (such as 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com