Laboratory diagnosis blood marker for liver fibrosis caused by hepatitis B virus
A hepatitis B virus, laboratory diagnosis technology, applied in the field of diagnostics, can solve problems such as application limitations, and achieve the effect of excellent diagnostic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Select the 25 cases and 75 healthy patients suffering from hepatitis B patients, classified according to the results FibroScan
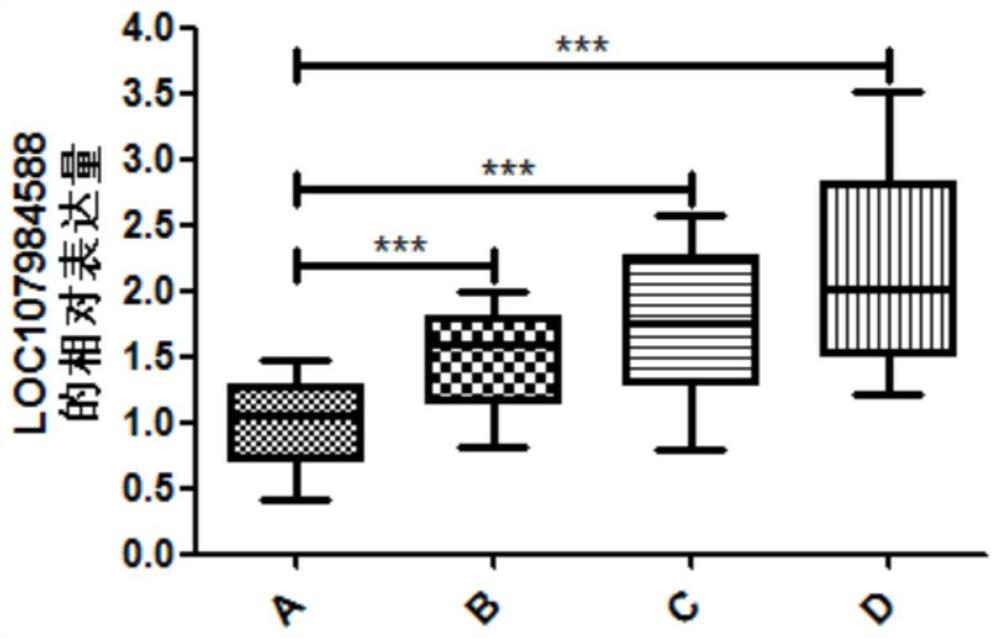
[0023] .. A. healthy control group, B group with mild to moderate fibrosis patients (n = 25), C severe liver fibrosis patients (n = 25), D early cirrhosis patients (25 cases).;
[0024] Patients Inclusion criteria: patient HBsAg and (or) HBV DNA positive for more than 6 months, now HBsAg positive and / or HBV DNA still positive; decompensated cirrhosis not entered, no tumor, no liver surgery, without anti history of viral therapy; patients aged 18-80 years, BMI less than equal to 28kg / m 2 .
[0025] Exclusion criteria: combined with other types of viral hepatitis, alcoholic liver disease, nonalcoholic fatty liver disease, autoimmune liver disease, AIDS, liver hydatid, drug-induced liver injury such as liver disease; the presence of decompensated cirrhosis, liver tumor or other neoplastic diseases; associated with liver fibrosis-related basic...
Embodiment 2
[0034] CDNA synthesized by reverse transcription
[0035] (1) arranged according to the following reaction system and the amount of reagent on ice:
[0036] Reagent name Usage amount 5 × GDNA ERASER BUFFER 2.0μL GDNA ERASER 1.0μL Total RNA 1.0ug RNASE FREE DH 2 O
[0037] (2) configure the reaction system, after heating 42 ℃ 2min, placed in temporary storage. 4 deg.] C;
[0038] (3) arranged according to the following reaction system and the amount of reagent on ice:
[0039] Reagent name Usage amount Step (1) reaction liquid 10.0μL 5 × primescript buffer 2 4.0μL Primescript RT Enzyme Mix i 1.0μL Rt Primer Mix 1.0μL RNASE FREE DH 2 O
[0040] (4) configured reaction system, 37 ℃ heating 15min, heated 5s after 85 ℃, placed in temporary storage 4 deg.] C.
Embodiment 3
[0042] Detecting the relative expression of each group LOC107984588
[0043] (1) Configuration of the reaction system and reagents used in the following amounts:
[0044] Reagent name Usage amount Sybr Green Premix EX TAQTM (2 ×) 10μL Upstream primer 0.4μL Downstream primer 0.4μL CDNA template 2μL DDH 2 O
7.2μL Total 20μL
[0045] The LOC107984588 sequence (see SEQ ID NO.1) Primer design:
[0046] LOC107984588 upstream primer sequence is: GAACCCTTCGCTTGGCAAAC, SEQ ID NO.2;
[0047] LOC107984588 downstream primer sequences: CTTAGCGGCTAAGCGTGAGT, SEQ ID NO.3;
[0048] The reaction conditions for PCR: 95 ℃ 10min, 1 cycle; 95 ℃ 20s, 60 ℃ 40s, 38 cycles; 60 ℃ 5min.
[0049] (2) 2 -△△Ct PCR methods for processing the data obtained, the results obtained are plotted chart.
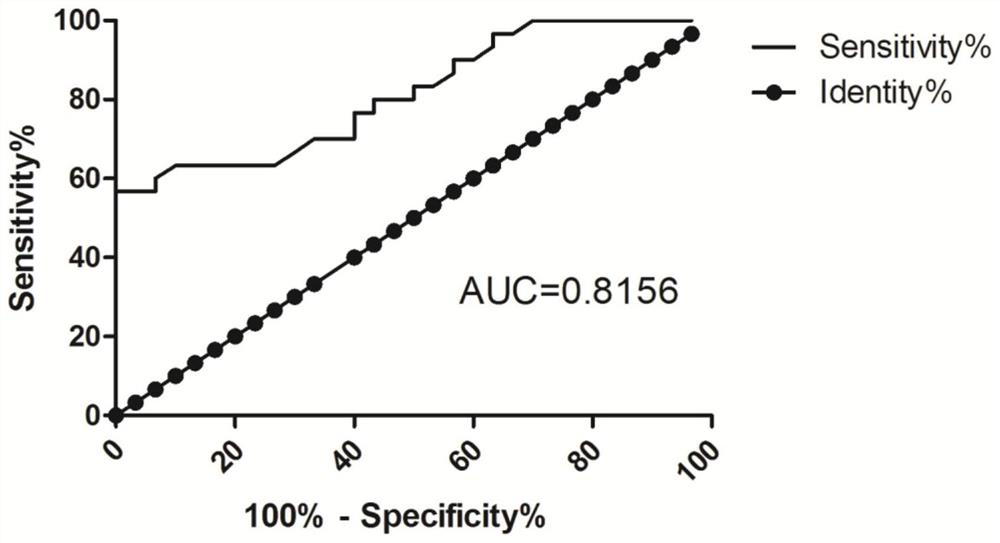
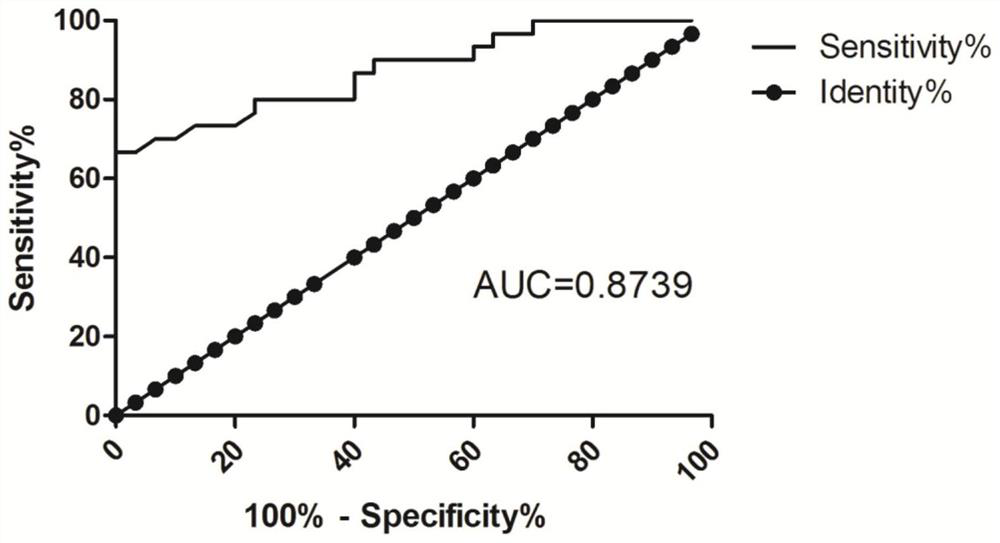
[0050] Experimental results figure 1 , The relative expression of the group B LOC107984588 was 1.48 ± 0.37, Group C relative expression LOC107984588 was 1.75 ± 0.54, the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com