Manganese phosphate nano material and rapid preparation method and application thereof
A manganese phosphate nanometer and rapid technology, applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of tissue and organ damage, low activation efficiency of immune cells, cytokine storm, etc., to achieve safe and controllable conditions, Good biocompatibility, good effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Take 250 μL of MnCl with a concentration of 1M 2 The solution was added into 4750 μL DMEM solution for reaction, and the reaction was carried out at 37° C. After 10 minutes of reaction, the solution turned into a turbid reaction liquid. The theoretical final concentration of manganese ions in the solution system is 50 mM.
[0038] 2) The reaction solution obtained in step 1) was centrifuged at 8000 rpm for 10 min, the precipitate was washed with deionized water and absolute ethanol, repeated three times, and dried at 50° C. to obtain spherical manganese phosphate nanomaterials of 30 nm.
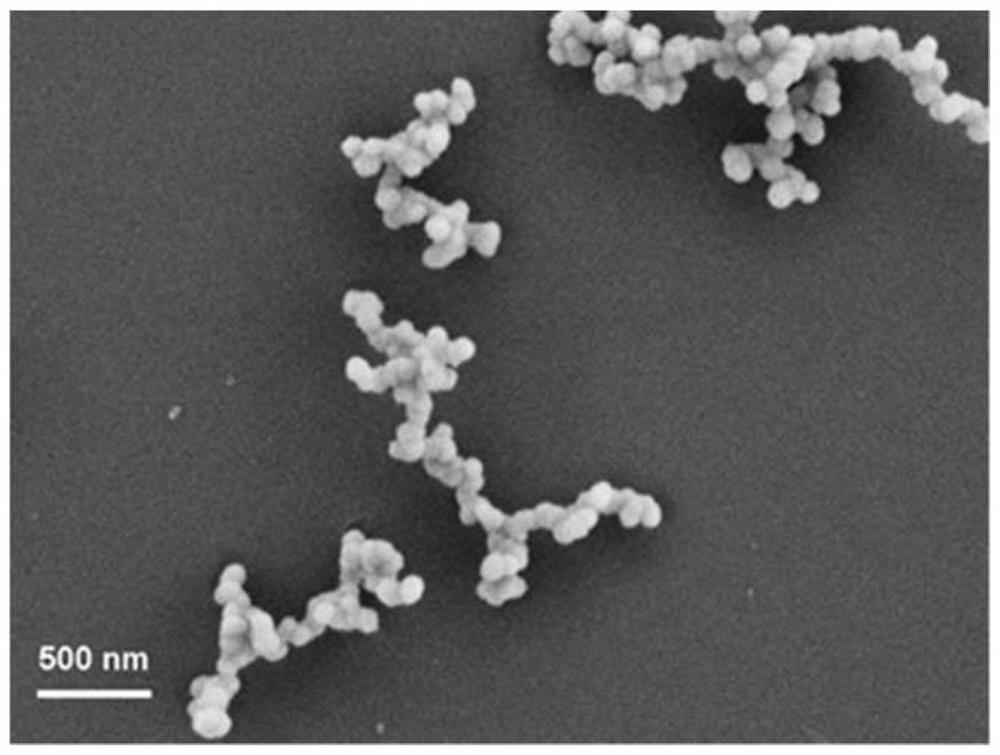
[0039]Gained spherical manganese phosphate nanomaterials are solid amorphous, and the particle diameter is 30nm, such as figure 1 , Figure 4 , Figure 7 Shown are its thermal field emission scanning electron microscope (FE-SEM) picture, transmission electron microscope (TEM) picture and energy spectrum analysis (EDS) picture, as figure 1 , Figure 4 As shown, thermal field emi...
Embodiment 2
[0041] 1) Take 5mL of MnCl with a concentration of 1M 2 The solution was added into 495 mL of DMEM solution for reaction, and the reaction was carried out at 37°C. After 1 hour of reaction, the solution turned into a turbid reaction liquid. The theoretical final concentration of manganese ions in the solution system is 10 mM.
[0042] 2) The reaction solution obtained in step 1) was centrifuged at 8000 rpm for 10 min, the precipitate was washed with deionized water and absolute ethanol, repeated three times, and dried at 50° C. to obtain 100 nm spherical manganese phosphate nanomaterials.
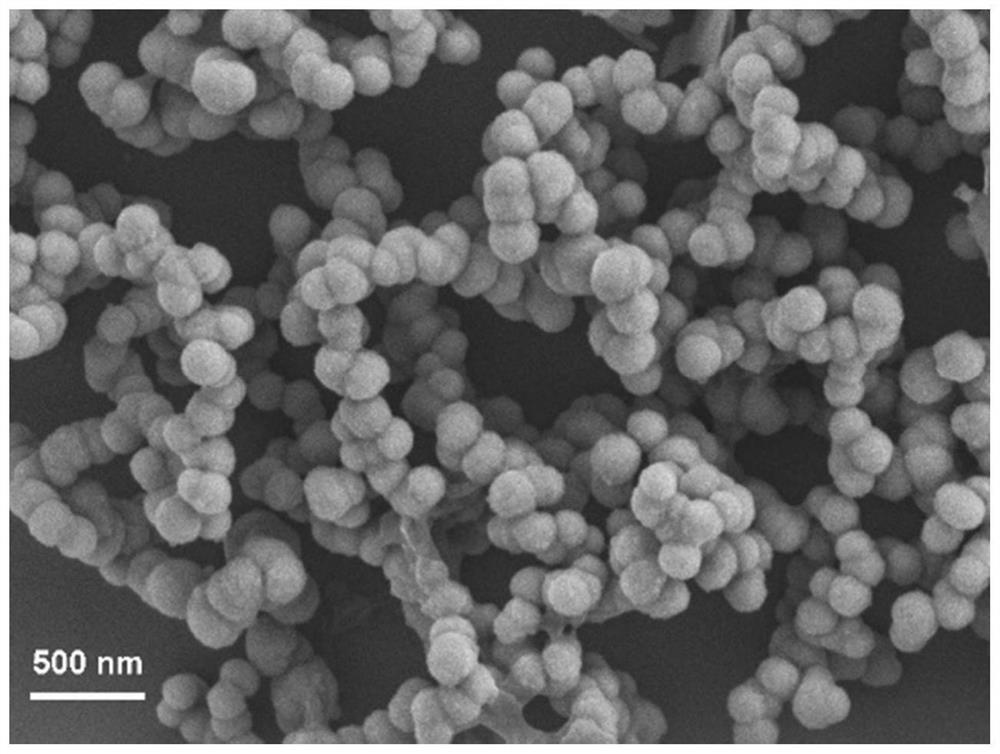
[0043] Gained spherical manganese phosphate nanomaterials are solid amorphous, and the particle diameter is 100nm, such as figure 2 , Figure 5 , Figure 8 Shown are their thermal field emission scanning electron microscope (FE-SEM) images, transmission electron microscope (TEM) images and energy dispersive spectroscopy (EDS) images, respectively. like figure 2 , Figure 5 As shown,...
Embodiment 3
[0045] 1) Take 10mL of MnCl with a concentration of 1M 2 Added into 490mL of DMEM solution for reaction, the reaction was carried out at 37°C, and after 2 hours of reaction, the solution turned into a turbid reaction liquid. The theoretical final concentration of manganese ions in the solution system is 20mM.
[0046] 2) Centrifuge the reaction solution obtained in step 1) at 8000 rpm for 10 min, wash the precipitate with deionized water and absolute ethanol, repeat three times, and dry at 50° C. to obtain 200 nm spherical manganese phosphate nanomaterials.
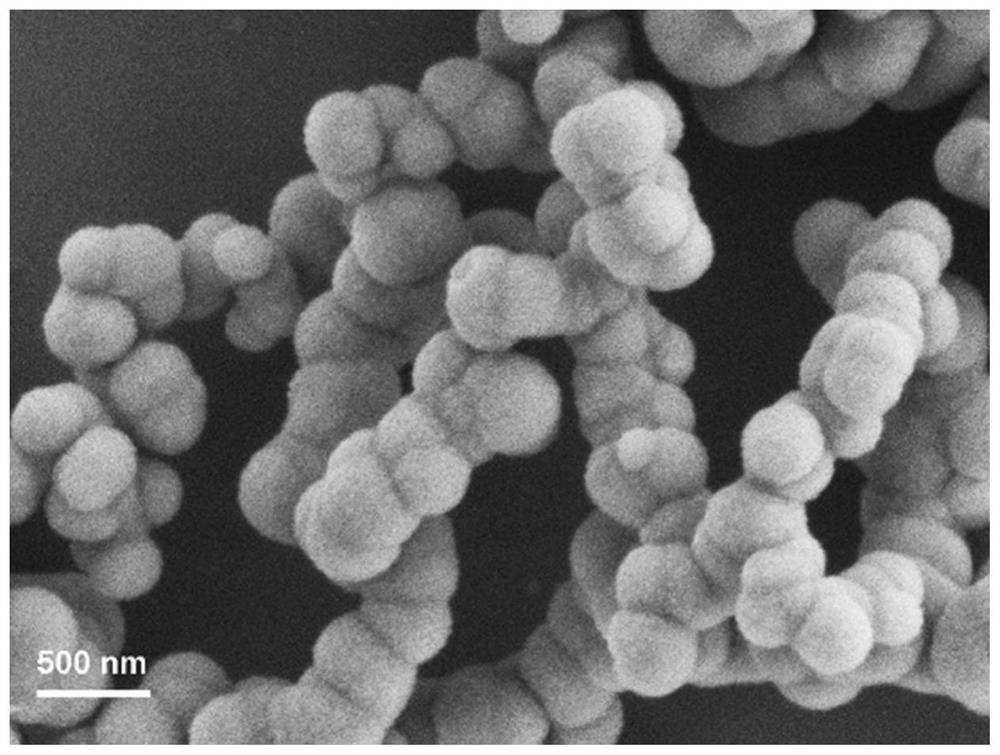
[0047] Gained spherical manganese phosphate nanomaterial is solid amorphous, and the particle diameter is 200nm, such as image 3 , Image 6 , Figure 9 Shown are their thermal field emission scanning electron microscope (FE-SEM) images, transmission electron microscope (TEM) images and energy dispersive spectroscopy (EDS) images, respectively. like image 3 , Image 6 As shown, thermal field emission scanning elect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com