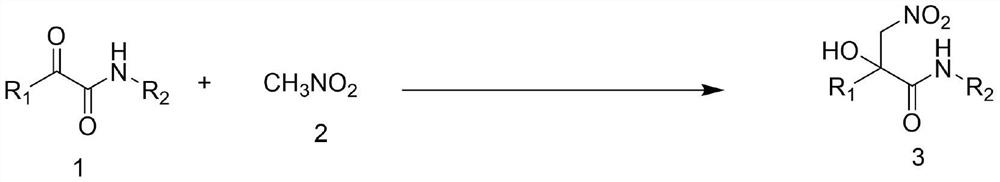
Synthesis method of alpha-hydroxyl beta-nitroamide compound
A synthesis method and technology of nitroamides, which are applied in the preparation of organic compounds, chemical instruments and methods, separation/purification of carboxylic acid amides, etc., can solve problems that have not been reported, and achieve environmental friendliness, wide sources, and cheap prices Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14
[0024] Embodiment 1-14 reaction condition optimization test
[0025] Using the α-ketoamide compound shown in formula 1a as the reaction substrate, and reacting with nitromethane to prepare the α-hydroxyl β-nitroamide compound shown in formula 3a, the effect of different synthesis conditions on the yield of the target product was discussed. The results are shown in Table 1. The reaction formula is as follows:
[0026]
[0027] Taking Example 10 as an example, the typical reaction operation is as follows:
[0028] Add α-ketoamide compound (0.1mmol), L-arginine (0.02mmol), nitromethane (2mmol) and water (1mL) successively to reactor shown in formula 1a, then place reactor in The reaction was stirred at room temperature for 6 hours. After the completion of the reaction was monitored by TLC, dichloromethane (30 mL*3) was added to the reaction mixture for extraction, the organic phases were combined, dried by adding anhydrous sodium sulfate, and concentrated in vacuo to obtain ...
Embodiment 15-36
[0039] Embodiment 15-36 reaction substrate expansion test
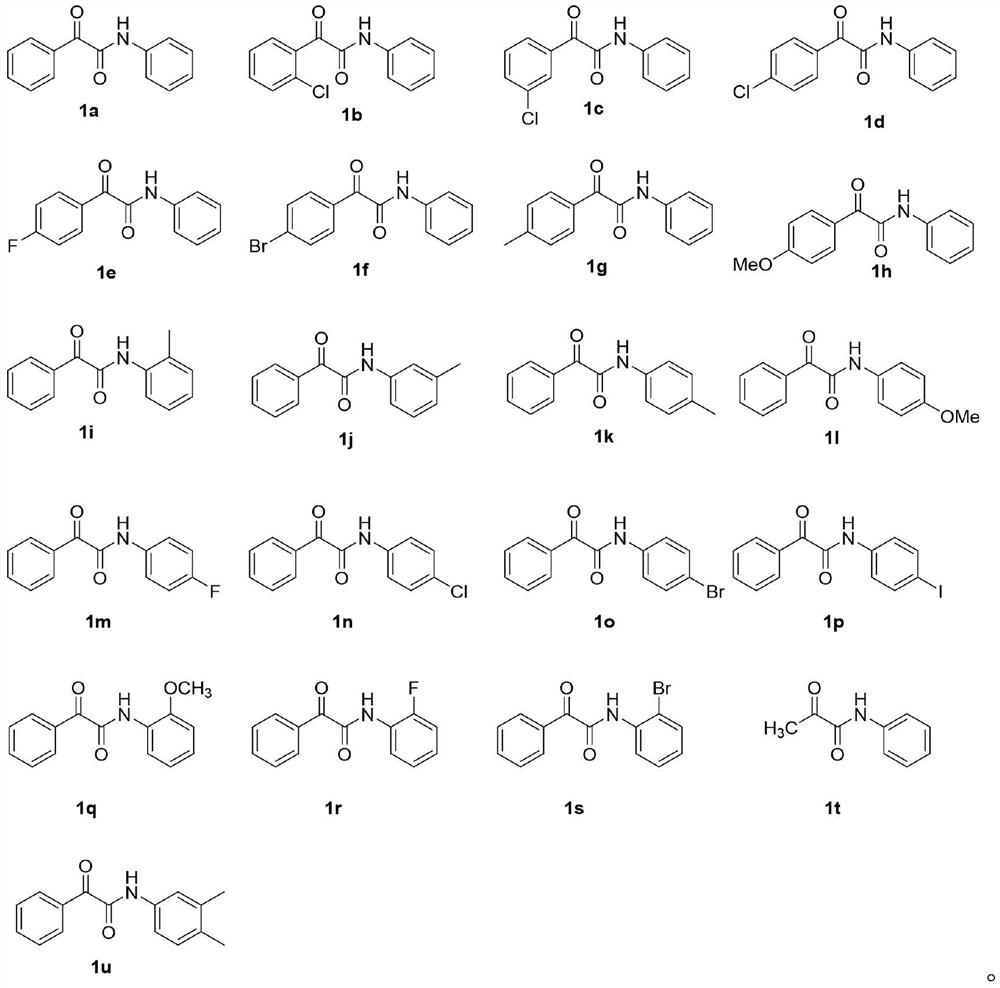
[0040] Taking the reaction conditions of Example 10 as the optimal reaction conditions, further explored the adaptability to the reaction substrate under the optimal reaction conditions, that is, only changing the type of reaction raw materials, and the rest of the reaction conditions and operations are the same as in Example 10, the results are as follows :
[0041]
[0042] Structural characterization of the product:
[0043] Compound 3b: white solid (31.0 mg, 97%). 1 H NMR (400MHz, CDCl 3 )δ8.30(s,1H),7.74–7.67(m,1H),7.62–7.54(m,2H),7.53–7.46(m,1H),7.45–7.33(m,4H),7.19(t, J=7.5Hz, 1H), 5.42(d, J=14.0Hz, 1H), 5.28(d, J=14.0Hz, 1H), 5.11(s, 1H). 13 C NMR (126MHz, CDCl 3 ) δ 167.16, 136.81, 133.49, 132.34, 132.12, 130.97, 129.12, 129.09, 127.77, 125.17, 120.12, 78.87.
[0044] Compound 3c: white solid (31.3 mg, 98%). 1 H NMR (500MHz, CDCl 3 )δ8.46(s,1H),7.72(s,1H),7.56-7.49(m,3H),7.37-7.30(m,4H),7.13(t,J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com