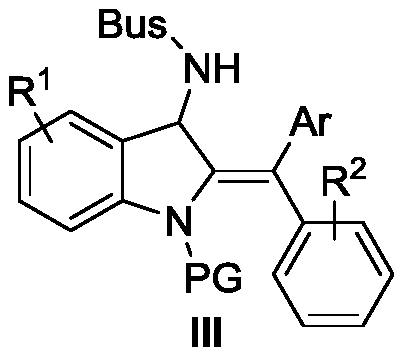
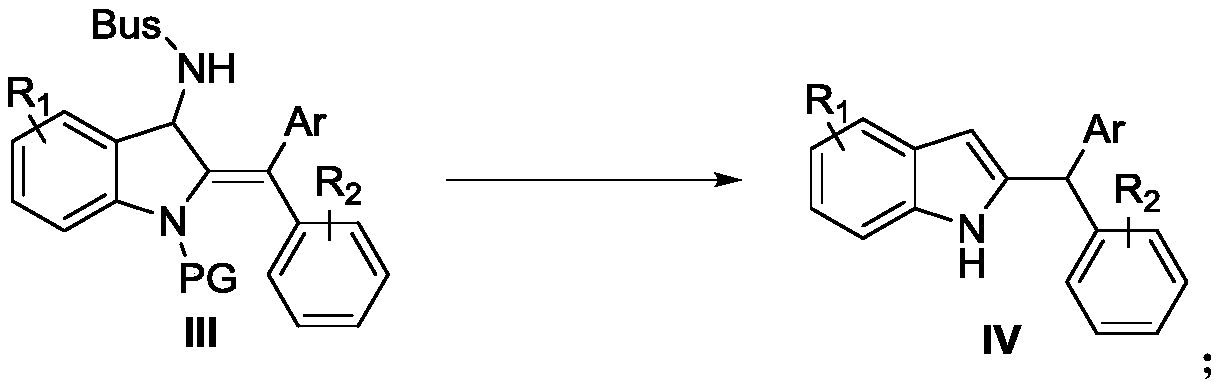
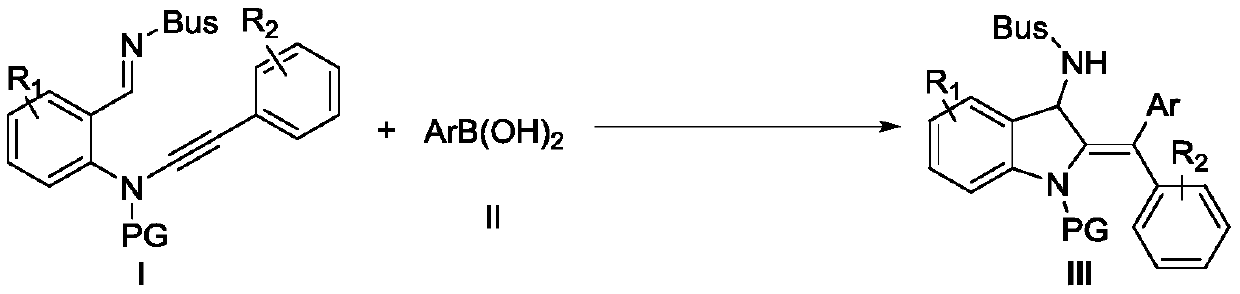2,3-disubstituted indoline compound as well as preparation method and application thereof
A compound, indoline technology, applied in organic chemistry, bulk chemical production, etc., can solve disclosure problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In the Schlenk capped reactor at room temperature, the imine-alkyne amine compound (0.1 mmol) protected by tert-butylsulfonyl (Bus) shown in formula I-1 and phenylboronic acid (0.2 mmol) shown in formula II were added successively. mmol), CuOTf (0.01mmol), the reactor was protected with argon, and anhydrous methanol (0.25mL) was added with a syringe, and then the reactor was placed at 50°C (oil bath) and stirred for 19h, and the reaction was completely monitored by TLC , followed by adding 0.01 mol of diethyl phthalate as an internal standard to the reaction solution, concentrating the reaction solution, taking a sample for nuclear magnetic detection, calculating the yield III-1 to be 50%, and the raw material hydrolyzate <1%.
Embodiment 2
[0057] The catalyst was replaced by CuI, and the reaction time was 48 hours until the raw materials were completely consumed. Other conditions were the same as in Example 1. The yield of III-1 NMR was 20%, and the yield of hydrolyzed products of raw materials was 28%.
Embodiment 3
[0059] Replacement catalyst is Cu(MeCN) 4 PF 6 , the reaction time is 44h until the raw materials are completely consumed, and the rest of the conditions are the same as in Example 1. The NMR yield of III-1 is 14%, and the hydrolyzed product yield of raw materials is less than 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com