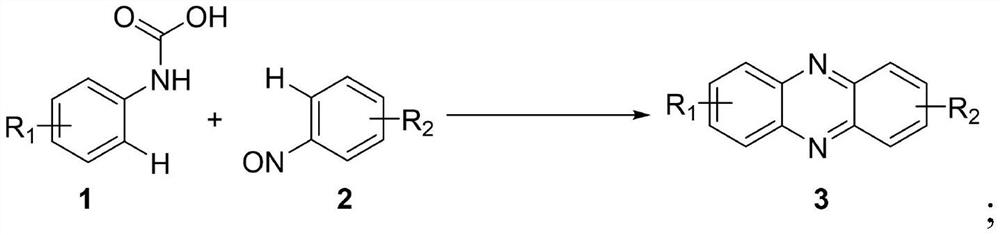
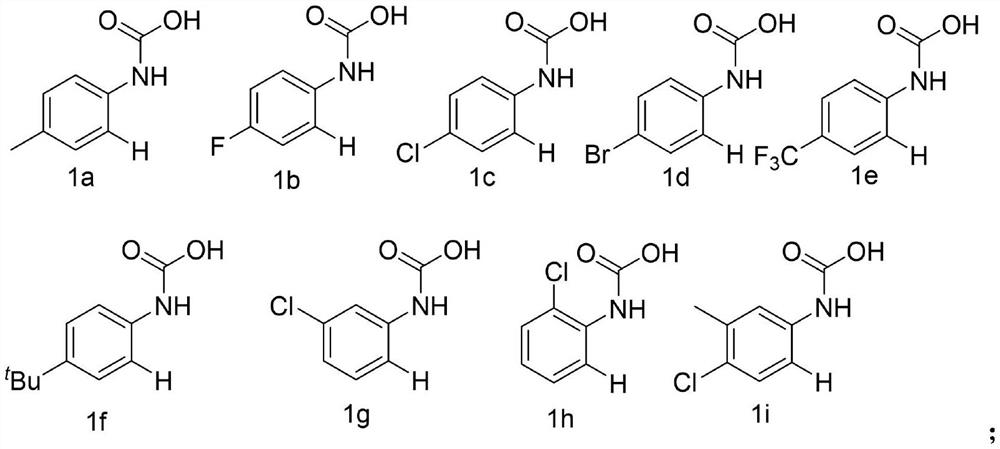
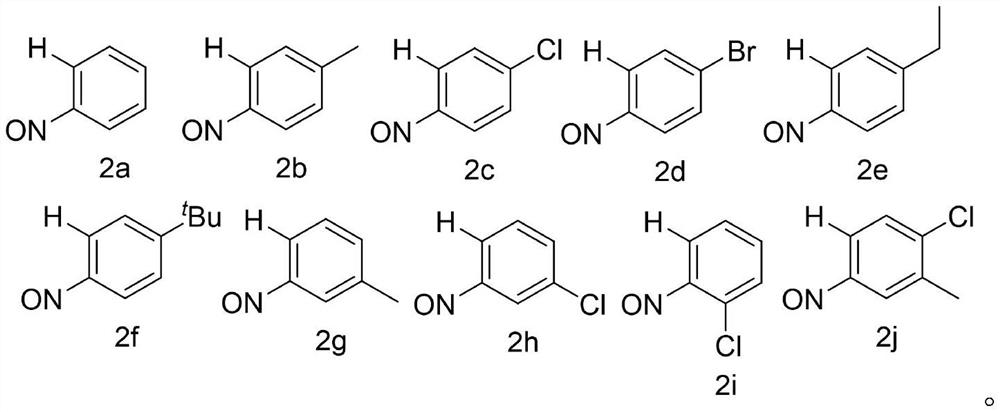
Preparation method of phenazine compound
A compound, phenazine technology, applied in the field of preparation of phenazine compounds, can solve problems such as lower-than-expected development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14
[0021] Examples 1-14 The reaction conditions optimization experiment
[0022] 1a of nitrosobenzene to p-type amino acid of formula 2a as the raw material, The effects of different reaction conditions on the catalytic yield of the target product 3aa, results as shown in Table 1, the following reaction formula:
[0023]
[0024] Table 1:
[0025]
[0026]
[0027] a Reaction conditions: 1a (0.1mmol), 2a (0.2mmol), solvent: (1.5mL), 140 ℃, under an air atmosphere in the reaction 16h, isolated yield. b PivOH (2equiv). c CF 3 SO 3 H (2equiv). d Solvent: ethyl acetate, e Solvent: dichloromethane, f The reaction under oxygen atmosphere 16h, g The reaction under a nitrogen atmosphere 16h, h 120 ℃, i 150 ℃. Wherein, TFE represents 2,2,2-trifluoroethanol.
[0028] Example 14 In an example, a typical test procedure is as follows:
[0029]
[0030] To 20mL Schlenk tube equipped with a magnetic stirring sealed reactor, were added successively p-amino-acid 1a (0.1mmol, 1.0equiv.), Nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com