Preparation method of infliximab freeze-dried preparation for injection
A technology for freeze-dried preparations and injections, which is applied in the field of preparation of infliximab freeze-dried preparations for injection, which can solve the problems of increased immunogenicity and multimer content, and achieve the reduction of polymer content and reduction of polymer content. content, risk reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
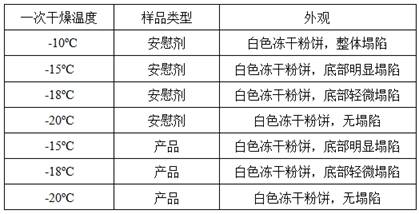
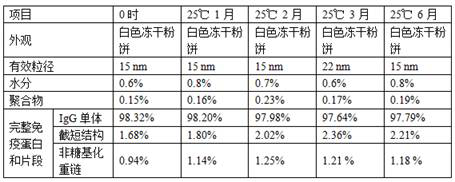
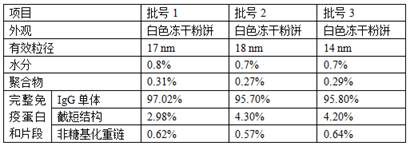
[0027] A method for preparing a freeze-dried preparation of infliximab for injection. The liquid before freeze-drying contains the following components: the concentration of infliximab is 20 mg / mL, the concentration of sodium phosphate is 10 mM, and the concentration of polysorbate 80 is 0.1 mg / mL, the sucrose concentration is 100mg / mL, and the pH value of the liquid medicine is 6.0.
[0028] Load the drug solution into the freeze dryer, keep in the freeze drying chamber at 5°C for 2h; cool down to -50°C, keep for 5h; vacuumize, sublimation and dry: heat up to -20°C once, keep for 50h, heat up to -20°C for the second time -10°C, keep for 8h; desorption: heat up to 20°C, keep for 10h, cool down to 5°C, keep for 20min.
Embodiment 2
[0030] A method for preparing a freeze-dried preparation of infliximab for injection. The liquid before freeze-drying contains the following components: the concentration of infliximab is 20 mg / mL, the concentration of sodium phosphate is 10 mM, and the concentration of polysorbate 80 is 0.1 mg / mL, the sucrose concentration is 100mg / mL, and the pH value of the liquid medicine is 5.8.
[0031] Load the drug solution into the freeze dryer, keep in the freeze drying chamber at 5°C for 2h; cool down to -40°C, keep for 8h; vacuumize, sublimation and dry: heat up to -20°C once, keep for 70h, heat up to -20°C for the second time 0°C, keep for 10h; desorption: heat up to 30°C, keep for 15h, cool down to 5°C, keep for 20min.
Embodiment 3
[0033] A method for preparing a freeze-dried preparation of infliximab for injection. The liquid before freeze-drying contains the following components: the concentration of infliximab is 20 mg / mL, the concentration of sodium phosphate is 10 mM, and the concentration of polysorbate 80 is 0.1 mg / mL, the sucrose concentration is 100mg / mL, and the pH value is 6.2.
[0034] Load the drug solution into the freeze dryer, keep it in the freeze drying chamber at 5°C for 2h; cool it down to -45°C, keep it for 6h; vacuumize, sublimation drying: raise the temperature to -20°C once, keep it for 60h, and raise the temperature twice to -8°C, keep for 9h; desorption: heat up to 25°C, keep for 12h, cool down to 5°C, keep for 20min.
[0035] The dosing method of medicinal liquid of the present invention:
[0036] A. Add water for injection into the solution bottle to keep the solution temperature at room temperature;
[0037] B. Add polysorbate 80, stir evenly, add sodium phosphate, stir unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com