Application of transferrin receptor inhibitor Ferristatin-II in preparation of medicine for treating cerebral trauma
A receptor inhibitor, transferrin technology, applied in the field of medicine, can solve problems such as unclear mechanism of action and no reports of Ferristatin-II, and achieve the effect of improving iron deposition, improving lipid peroxidation, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
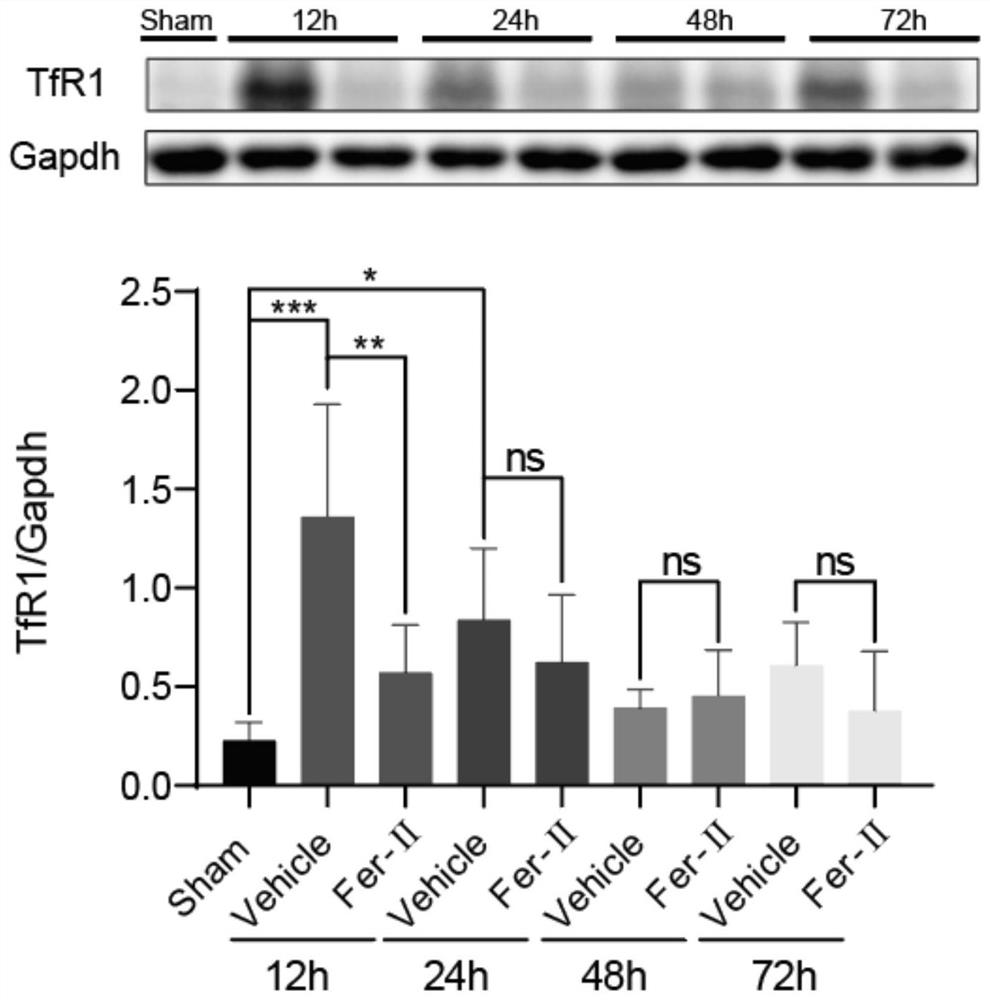
[0042] (1) Drug preparation and administration method: Fer-II (Sigma Company, product number: C1144) was prepared at a concentration of 10 mg / kg with reference to the previous research basis and preliminary experimental results. The preparation method is as follows: according to the instructions, the Fer-II is prepared to the required concentration with medical physiological saline. Fer-II was administered by intraperitoneal injection 30 minutes to 1 hour after TBI, and administered once every 12 hours until the day before execution.
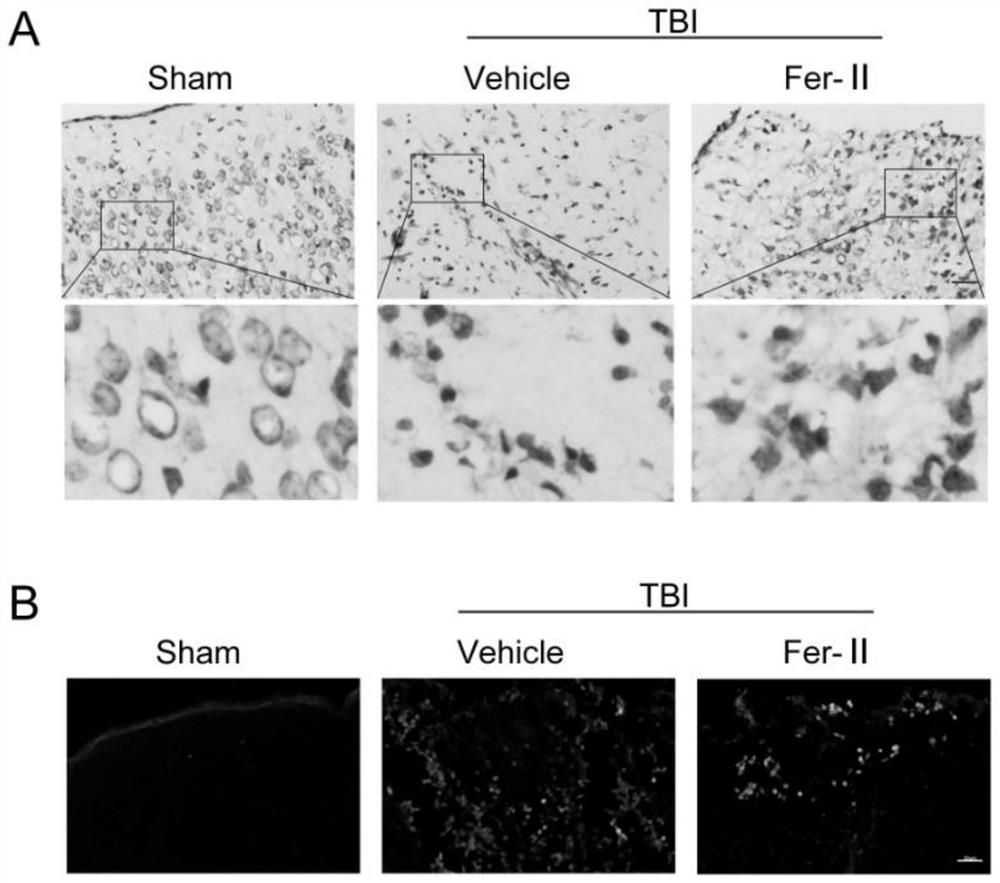
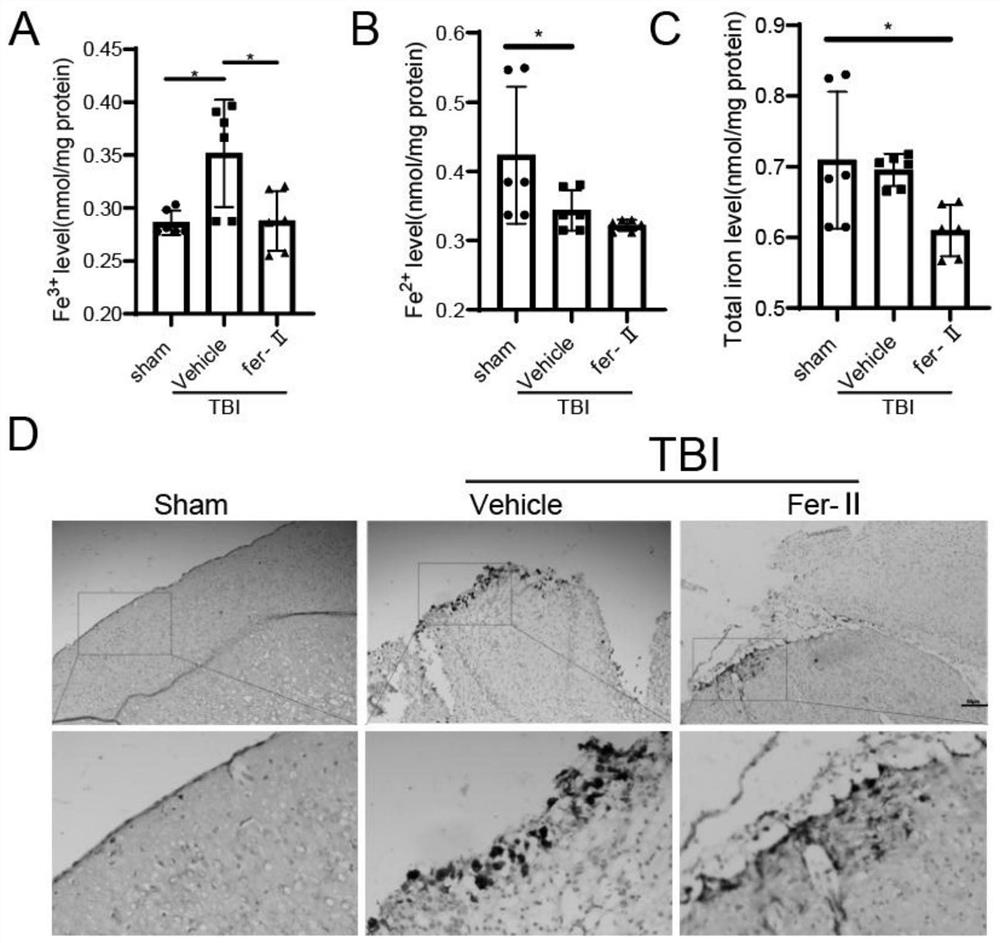
[0043] (2) Experimental grouping: There are the following five situations: ① TfR1 time-course change experiment after TBI, 6-week-old ICR mice were randomly divided into 9 groups, 6 in each group, grouped as: Sham, 12h, 12h+Fer-II , 1d, 1d+Fer-II, 2d, 2d+Fer-II, 3d and 3d+Fer-II. ② Nissl, FJB staining, Prussian blue and non-heme iron detection experiments, 6-week-old ICR mice were randomly divided into 3 groups, 6 mice in each group, and the gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com