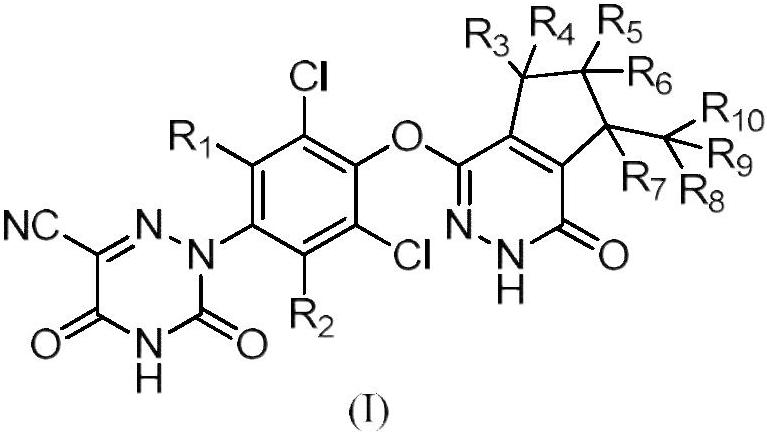
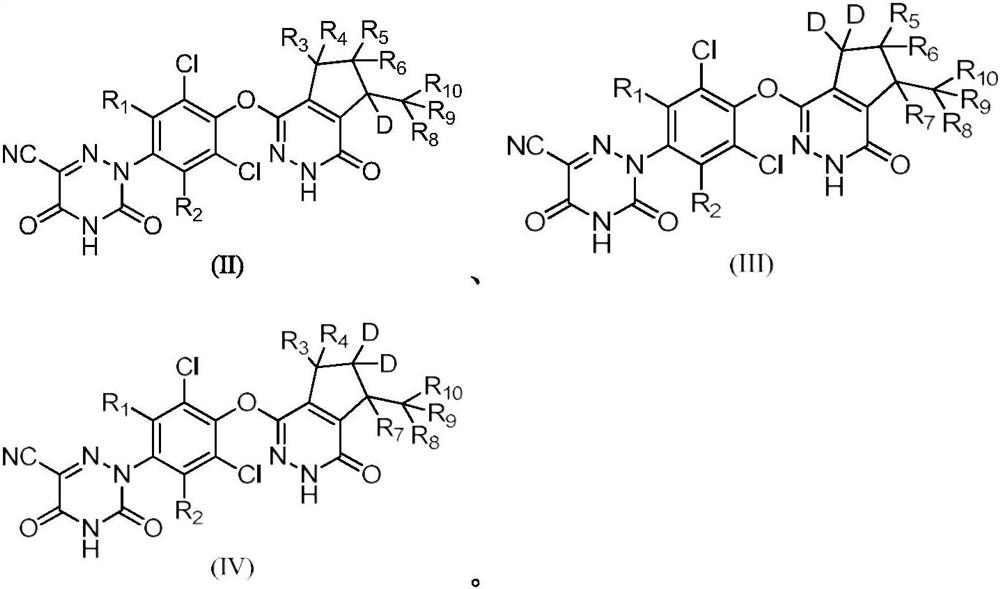
Deuterated pyridazinone compound and application thereof
A technology of compounds and uses, which is applied in the field of preparation of drugs for the treatment of metabolic-related diseases, and can solve problems such as restrictions on the use of thyroid hormones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
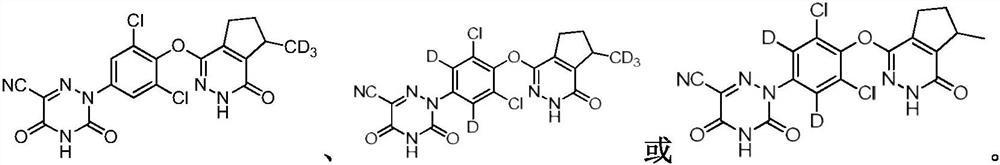
[0064] (R)-2-(3,5-dichloro-4-((7-(methyl-d3)-1-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[d] Pyridazin-4-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (Compound 1)
[0065] (R)-2-(3,5-dichloro-4-((7-(methyl-d3)-1-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[d]pyridazin-4-yl )oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitril (compound 1)
[0066]
[0067]The first step: ethyl 1-(methyl-d3)-2-oxocyclopentane-1-carboxylate (1b)
[0068] ethyl 1-(methyl-d3)-2-oxocyclopentane-1-carboxylate(1b)
[0069] Add 1a (0.65Kg, 4.16mol) in a 10L three-necked flask, dissolve in acetone (6000mL), add potassium carbonate (2.00Kg, 3.32mol) while stirring, and add deuteroiodomethane (1.80Kg, 12.41mmol) dropwise at room temperature , stirred at room temperature for 100 h after dropping, filtered with suction, concentrated, and separated by column chromatography (petroleum ether: ethyl acetate (v / v) = 10:1) to obtain the title compound 1b (580.10 g, 90%).
...
Embodiment 2
[0106] (R)-2-(3,5-dichloro-4-((7-methyl-1-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[d]pyridazine-4 -yl)oxyl]phenyl-2,6-d2)-3,5-dioxa-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (compound 2 )
[0107] (R)-2-(3,5-dichloro-4-((7-methyl-1-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[d]pyridazin-4-yl)oxy)phenyl -2,6-d2)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (compound 2)
[0108]
[0109] The first step: 1-(5-methylcyclopent-1-en-1-yl)pyrrolidine (2c)
[0110] 1-(5-methylcyclopent-1-en-1-y1)pyrrolidine (2c)
[0111] 2a (6.05g, 61.22mmol) was dissolved in toluene (70mL), 2b (6.53g, 91.84mmol) and p-toluenesulfonic acid monohydrate (1.16g, 6.12mmol) were added, and the reaction was carried out under reflux at 130°C for 16h. Cool to room temperature, and distill under reduced pressure to obtain the title compound 2c (5.01 g, 65%). No purification required.
[0112] The second step: 1,4-dichloro-5-methyl-6,7-dihydro-5H-cyclopenta[d]pyridazine (2e)
[0113] 1,4-dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com