Mutant gene related to hereditary glomerular diseasse, and application thereof
A technology for glomerular diseases and mutation genes, applied in the field of human genetics and internal medicine and cardiovascular, to achieve the effect of reducing the birth of children with diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 - Mutated Genes Associated with Inherited Glomerular Diseases
[0018] The mutated genes associated with hereditary glomerular diseases, the specific mutations are shown in Table 1 below:
[0019] Table 1 Specific detection results of mutated genes associated with hereditary glomerular diseases
[0020] Gene genomic location transcript number base change amino acid changes Reference Genome Version exon number FN1 chr2:216273077 NM_001306129 c.2372A>G p.Tyr791Cys GRCh37 / hg19 Exon16
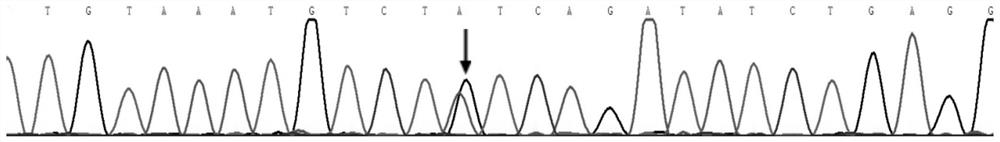
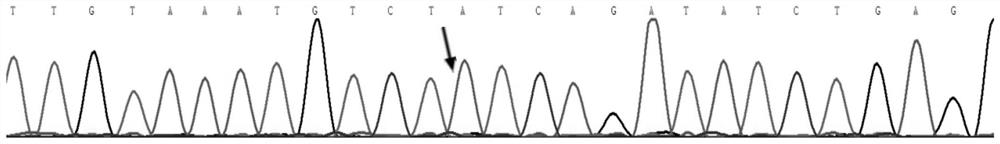
[0021] (1) At genomic position chr2:216273076-chr2:216273125, the sequence of the wild-type FN1 gene is:
[0022] TGAACATCCCTGACCTGCTTCCTGGCCGAAAATACATTGTAAATGTCT T, It is the base at chr2:216273077 of the wild-type FN1 gene in the genome.
[0023] At the corresponding genomic location, the sequence of the mutated gene associated with the inherited glomerular disease is:
[0024] TGAACATCCCTGACCTGCTTCCTGGCCGAAAATACATTGTAAATGTCT T,...
Embodiment 2
[0033] Example 2 - Detection Kit for Mutated Genes Associated with Hereditary Glomerular Diseases
[0034] Detection kits for mutated genes related to hereditary glomerular diseases, including Taq DNA polymerase, PCR buffer and primers, etc. The specific primers are as follows:
[0035] Upstream primer (FN1-E16F, SEQ ID NO: 1): 5' AGCACATTACCTTTCTAGTCGCTT 3";
[0036] Downstream primer (FN1-E16R, SEQ ID NO:2): 5'AACTTGGTCCACAGTCGTGTC 3';
[0037] Length: 394bp.
[0038] The specific steps of using this kit to screen the mutated disease-causing gene FN1 are as follows: extract the DNA of the test subject, and then use the designed primer combination (SEQ ID NO:1 and SEQ ID NO:2) to amplify the FN1 gene to obtain For the PCR product, use 1.5% agarose gel electrophoresis to detect the PCR product, select 1000bp Marker as a reference, check and verify that the amplified product is the expected size, and finally sequence the PCR product. Obtained reference sequences from the NC...
Embodiment 3
[0039] Embodiment 3-family verification experiment
[0040] In this example, the method of family linkage analysis is used to verify the pathogenicity of the mutated genes related to hereditary glomerular diseases.
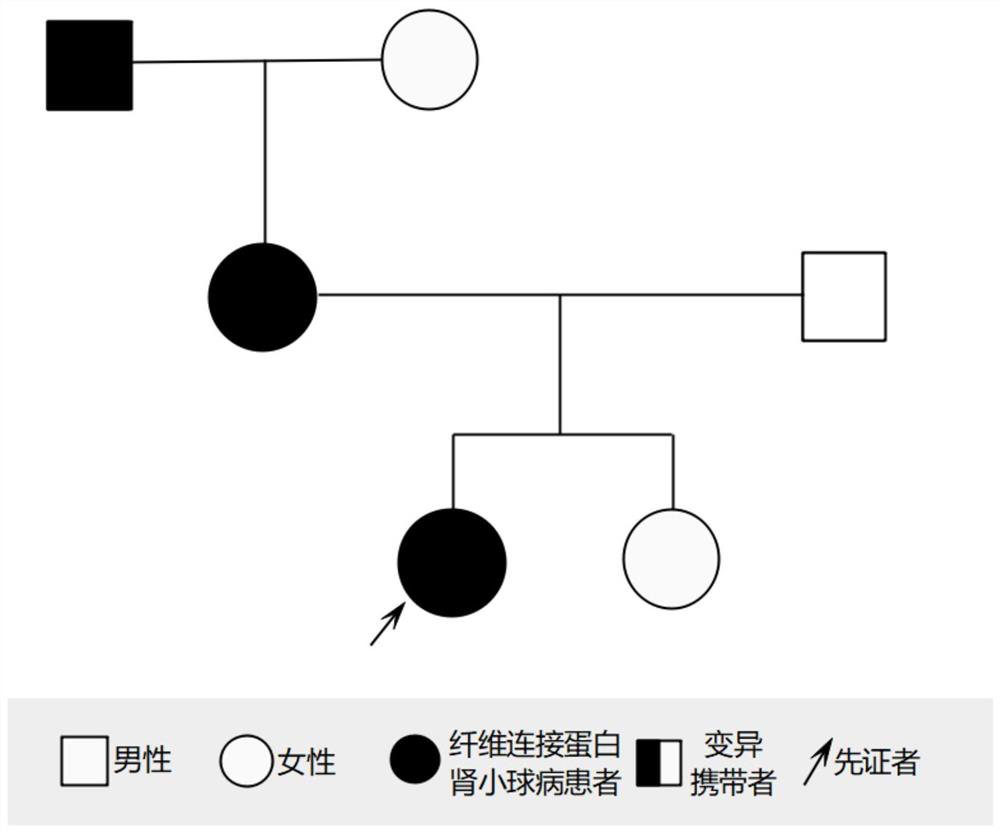
[0041] Specifically, three generations of members of a family with familial fibronectin glomerulopathy were selected, and the proband (female, 15 years old) in this family was clinically diagnosed with fibronectin glomerulopathy.
[0042] On the premise that the proband and his family members voluntarily sign the informed consent, 5-10mL whole blood samples will be sent, and a medical record database will be established to record the proband's condition and family status in detail. This study has been approved by the institutional ethics committee.
[0043] Description of the clinical profile of the proband:
[0044] Table 3 Clinical profile of the proband
[0045]
[0046]
[0047] The detection kit provided in Example 2 was used to carry out genetic det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com