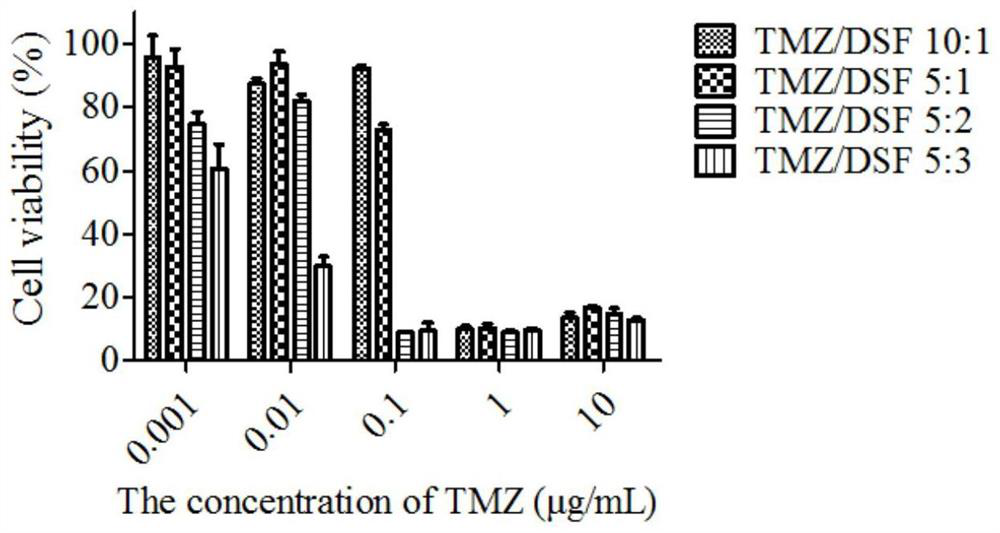
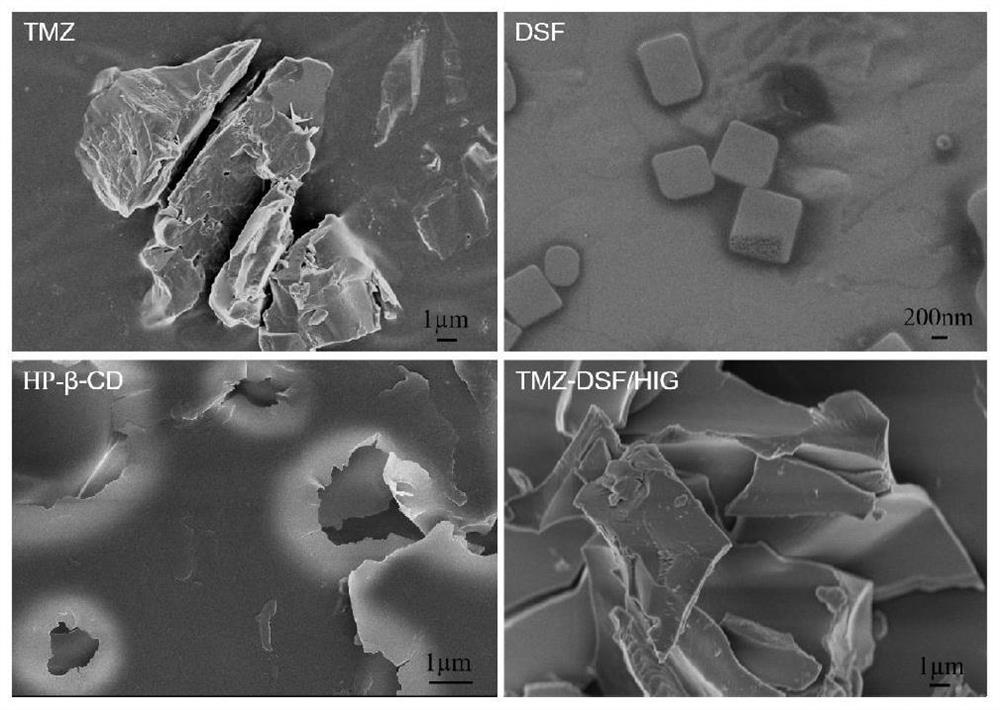
Ion-sensitive nasal in-situ gel carrying temozolomide and disulfiram as well as preparation method and application of ion-sensitive nasal in-situ gel
A temozolomide and ion-sensitive technology, applied in the field of medicine, can solve the problems of low oral bioavailability, poor fat solubility, water solubility, and less brain drug aggregation, so as to achieve good biocompatibility and safety, and overcome drug resistance Sex, the effect of improving drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] In yet another specific embodiment of the present invention, the preparation method of the above-mentioned pharmaceutical preparation is provided. Specifically, the pharmaceutical preparation is an ion-sensitive nasal in-situ gel, and its preparation method includes:
[0050] (1) Completely dissolving the clathrate material in the aqueous solution containing the gelling agent material, and obtaining the first mixed solution after ultrasonication;
[0051] (2) adding an organic solution containing disulfiram to the first mixed solution obtained in step (1) to obtain a second mixed solution;
[0052] (3) Add temozolomide to the second mixture, heat and stir until the two drugs are completely dissolved, the organic solvent is volatilized, and centrifuged to obtain an ion-sensitive in-situ nasal gel containing temozolomide and disulfiram.
[0053] It should be noted that the ion-sensitive nasal in-situ gel prepared in step (3) of the present invention can be further made in...
Embodiment 1
[0064] A preparation method for an ion-sensitive nasal in-situ gel carrying temozolomide and disulfiram, comprising the steps of:
[0065] Add 50 mg of temozolomide TMZ to 300 mg / mL hydroxypropyl-β-cyclodextrin HP-β-CD aqueous solution containing 0.4% deacetylated gellan gum DGG, and then add 300 μL of acetonitrile solution of 30 mg disulfiram DSF. Under the condition of heating at 40°C and stirring at 600rpm, TMZ and DSF are gradually clathrated and dissolved. After 2 hours, when TMZ and DSF were completely clathrated and acetonitrile was completely volatilized, an ion-sensitive nasal in-situ gel containing temozolomide and disulfiram with excellent gelling ability was obtained.
Embodiment 2
[0067] A preparation method for an ion-sensitive nasal in-situ gel carrying temozolomide and disulfiram, comprising the steps of:
[0068] 300 μL of an acetonitrile solution of 30 mg DSF was added to a 300 mg / mL aqueous solution of HP-β-CD containing 0.4% DGG, and then 50 mg of TMZ was added. Under the condition of heating at 40°C and stirring at 400rpm, TMZ and DSF are gradually clathrated and dissolved. After 2 hours, when TMZ and DSF were completely clathrated and acetonitrile was completely volatilized, an ion-sensitive nasal in-situ gel containing temozolomide and disulfiram with excellent gelling ability was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com