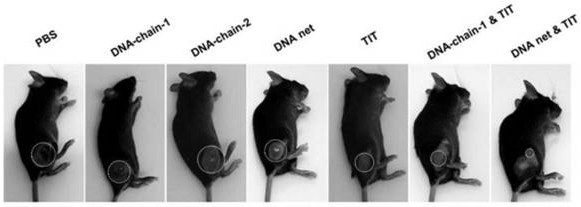
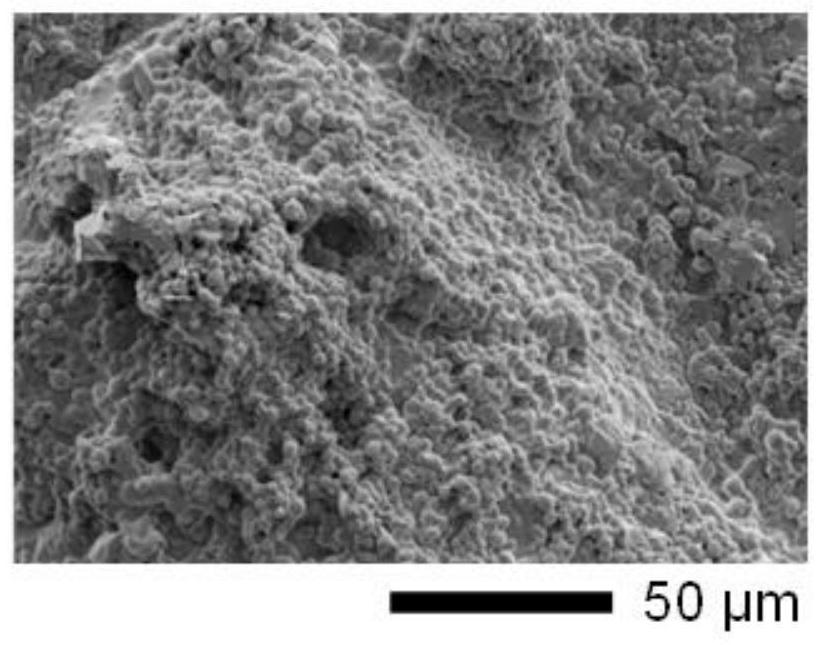
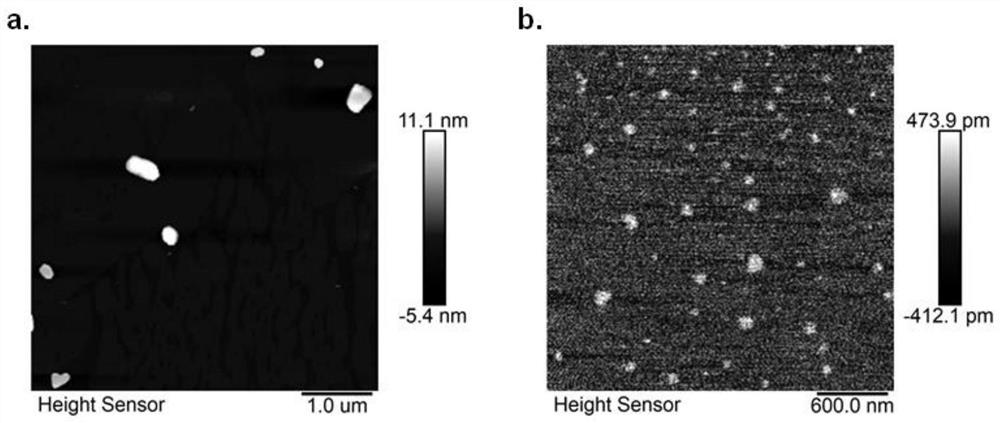
DNA network hydrogel, preparation method and composition thereof
A hydrogel and composition technology, applied in the field of biopharmaceuticals, can solve the problems of high cost, autoimmune inflammation, etc., and achieve the effect of significant immune curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for preparing a DNA network hydrogel encapsulating T lymphocytes, comprising the steps of:
[0036] DNA network hydrogel preparation
[0037] a. Design the antisense sequence (as shown in SEQIDNO.6) of the PD-1 DNA aptamer on the circular DNA template one, and design a DNA sequence complementary to a part of the circular DNA template on the circular DNA template two;
[0038] b. Proportionally, divide 5×10 -3 nmol of circular DNA template 1, 3U of phi29 DNA polymerase at a final concentration of 1×phi29 DNA polymerase buffer, a final concentration of 2×BSA, 0.05nmol of dNTPs, and a final concentration of 60mmol / L of NaCl in sterile water Make up to 100 μL; shake at 37°C for 4 hours at 350 rpm to obtain RCA product 1;
[0039] c. Proportionally, divide 5×10 -3nmol of circular DNA template II, 3 U of phi29 DNA polymerase at a final concentration of 1× phi29 DNA polymerase buffer, a final concentration of 2× BSA, 0.05 nmol of dNTPs, a final concentration of 60 ...
Embodiment 2
[0072] A method for preparing a DNA network hydrogel encapsulating T lymphocytes, comprising the steps of:
[0073] DNA network hydrogel preparation
[0074] a. Design the antisense sequence of the PD-1 DNA adapter (as shown in SEQ ID NO.6) to the circular DNA template one, and design a DNA sequence complementary to a part of the circular DNA template on the circular DNA template two ;
[0075] b. Proportionally, divide 1×10 -2 nmol of circular DNA template 1, 3.5U of phi 29 DNA polymerase, final concentration of 1×phi 29 DNA polymerase buffer, final concentration of 2×BSA, 0.06nmol of dNTPs, final concentration of 70mmol / L NaCl, with Make up to 100 μL with sterile water; shake at 36°C for 16 hours at 450 rpm to obtain RCA product 1;
[0076] c. Proportionally, divide 5×10 -3 ~1×10 -2 nmol of circular DNA template II, 3.5U of phi 29 DNA polymerase, final concentration of 1×phi 29 DNA polymerase buffer, final concentration of 2×BSA, 0.06nmol of dNTPs, final concentration o...
Embodiment 3
[0106] A method for preparing a DNA network hydrogel encapsulating T lymphocytes, comprising the steps of:
[0107] DNA network hydrogel preparation
[0108] a. Design the antisense sequence of the PD-1 DNA adapter (as shown in SEQ ID NO.6) to the circular DNA template one, and design a DNA sequence complementary to a part of the circular DNA template on the circular DNA template two ;
[0109] b. Proportionally, the 8×10 -3 nmol of circular DNA template 1, 4U of phi 29 DNA polymerase, final concentration of 1×phi 29 DNA polymerase buffer, final concentration of 2×BSA, 0.08nmol of dNTPs, final concentration of 80mmol / L NaCl, with no Make up to 100 μL with bacterial water; shake at 36°C for 8 hours at 400 rpm to obtain RCA product 1;
[0110] c. Proportionally, divide 5×10 -3 nmol of circular DNA template II, 3U of phi29 DNA polymerase at a final concentration of 1×phi 29 DNA polymerase buffer, a final concentration of 2×BSA, 0.05nmol of dNTPs, and a final concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com