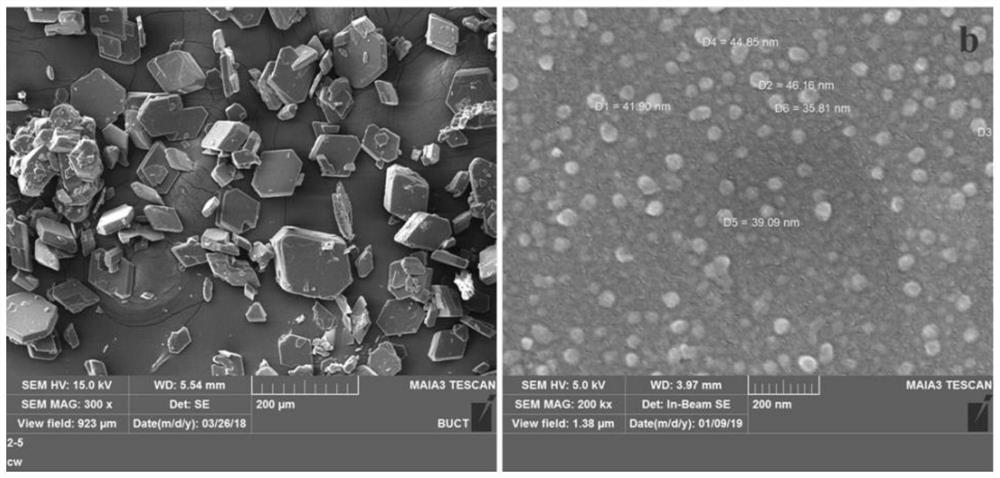
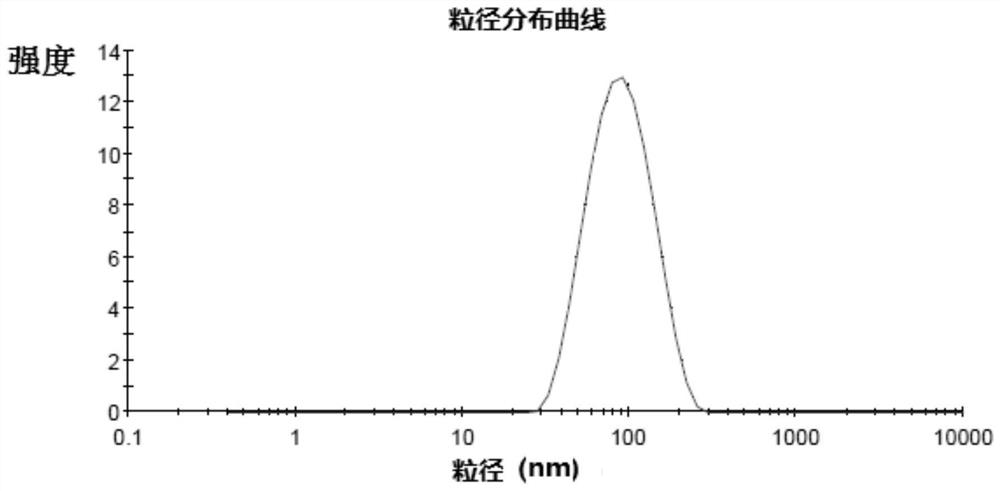
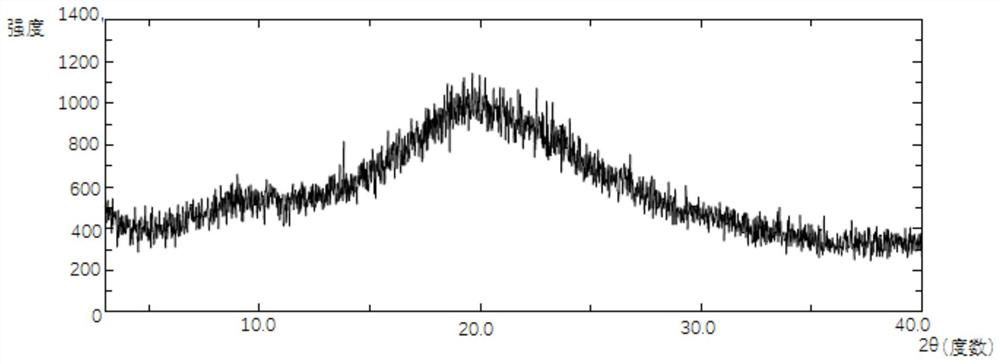
Flucoladine nano-micelle preparation as well as preparation method and application thereof
A nano-micelle and preparation technology, which is applied in the field of medicine, can solve problems such as poor safety, and achieve the effects of increased bioavailability, high bioavailability, and increased blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of Fluocoridine Nanomicelle Oral Liquid
[0071] Flucoridine raw material 2.4g is dissolved in the 0.2mol / L sodium hydroxide aqueous solution of 120mL, and polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer 4.8g and 4.8g poloxa Mu 407 was dissolved in 480 mL of 0.05 mol / L hydrochloric acid aqueous solution. Under high-speed stirring at 1000 rpm, the sodium hydroxide solution mixed with flucoridine was added to the hydrochloric acid aqueous solution of the polymer through a peristaltic pump, and then continued to stir for 20 minutes. Then centrifuge at 10,000 rpm for 20 minutes to remove the precipitate, and the supernatant is the fluocoridine micelle solution. The concentration of flucoridine was 3.6 mg / mL. The resulting solution was ultrafiltered in an ultrafiltration cup with a regenerated cellulose filter membrane (100KDa), to obtain a higher concentration of fluocladine micellar solution, the concentration of flucoridine was ...
Embodiment 2
[0076] Preparation of Fluocoridine Micellar Concentrate
[0077] Dissolve 2 g of flucoridine raw material in 140 mL of acetone, and dissolve 20.0 g of polyethylene glycol 15 hydroxystearate and 5.0 g of tocopheryl polyethylene glycol succinate in 500 mL of purified water. Under 1000 rpm high-speed stirring by a peristaltic pump, the acetone solution dissolved with flucoridine is added dropwise to the solution of polyethylene glycol 15 hydroxystearate and tocopheryl polyethylene glycol succinate, dropwise Stirring was then continued for 20 minutes after the addition was complete. Then place the reaction solution in a ventilated place and stir at 200 rpm to volatilize the acetone in the reaction solution for more than 36 hours until the acetone is completely volatilized. The remaining solution is centrifuged at 20°C and 12000 rpm for 20 minutes to remove the precipitate and the supernatant The solution is the micellar solution of flucoridine. The concentration of flucoridine...
Embodiment 3
[0079] Preparation of Fluocoridine Micellar Concentrate
[0080] Dissolve 1.0 g of flucoridine in 70 mL of acetone, and dissolve 20.0 g of polyethylene glycol 15 hydroxystearate and 0.25 g of sodium lauryl sulfate in 200 mL of purified water. Under high-speed stirring at 1000 rpm, the acetone solution dissolved with flucoridine is added dropwise to the solution of polyethylene glycol 15 hydroxystearate and sodium lauryl sulfate through a peristaltic pump. Stirring was then continued for 10 minutes. Then place the reaction solution in a ventilated place and stir at 300 rpm to volatilize the acetone in the reaction solution for more than 24 hours until the acetone is completely volatilized. The remaining solution is centrifuged at 20°C and 12000 rpm for 20 minutes to remove the precipitate and the supernatant That is flucoridine micellar solution. The concentration of flucoridine was 5 mg / mL. Using regenerated cellulose filter membrane (100Kda) for membrane filtration and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com