Synthesis method of desmopressin acetate dimer impurity
A technology for desmopressin and its synthesis method, which is applied in the field of synthesis of desmopressin acetate dimer impurities, can solve the problems of no specificity, low yield, cumbersome liquid phase synthesis, etc., and achieve cost saving , improved yield and purity, and the effect of avoiding linear peptide oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
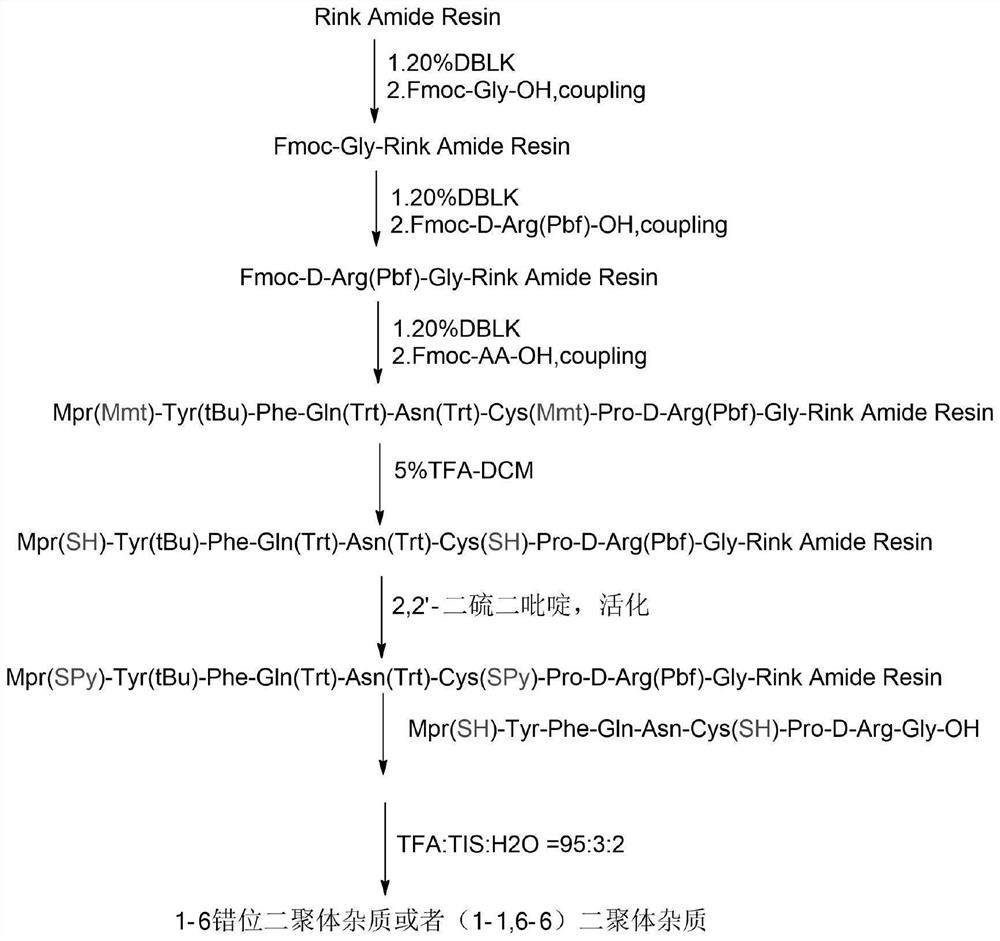
[0062] Such as figure 1 As shown, it is a synthetic route diagram of (1-6) cross dimer or (1-1,6-6) parallel dimer impurity in Example 1 of the present invention, and the specific synthetic route includes the following steps:
[0063] Peptide Resin Synthesis:
[0064] Weigh 11.9 g of RinkAmide resin (substitution degree is 0.42 mmol / g, 5 mmol), and sequentially couple Fmoc-Gly-OH, Fmoc-D-Arg(Pbf)-OH, Fmoc-Pro-OH, Fmoc-Cys(mmt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Phe-OH, Fmoc-Tyr(tBu)-OH, Mpr(mmt)-OH. Finally, DMF was washed 3 times, DCM was washed 3 times, and methanol was shrunk to obtain 24.1 g of peptide resin. The obtained 16g peptide resin was treated with TFA:EDT:H 2 O=95:2.5:2.5 system was cleaved to obtain 1.2 g of linear peptides.
[0065] Synthesis of dimers:
[0066] Take 8g of peptide resin, add DCM to swell for 5 minutes, drain, then add DCM to swell for 5 minutes; repeat 2 times, drain, then add 120mL of 5% trifluoroacetic acid-DCM solution, react f...
Embodiment 2
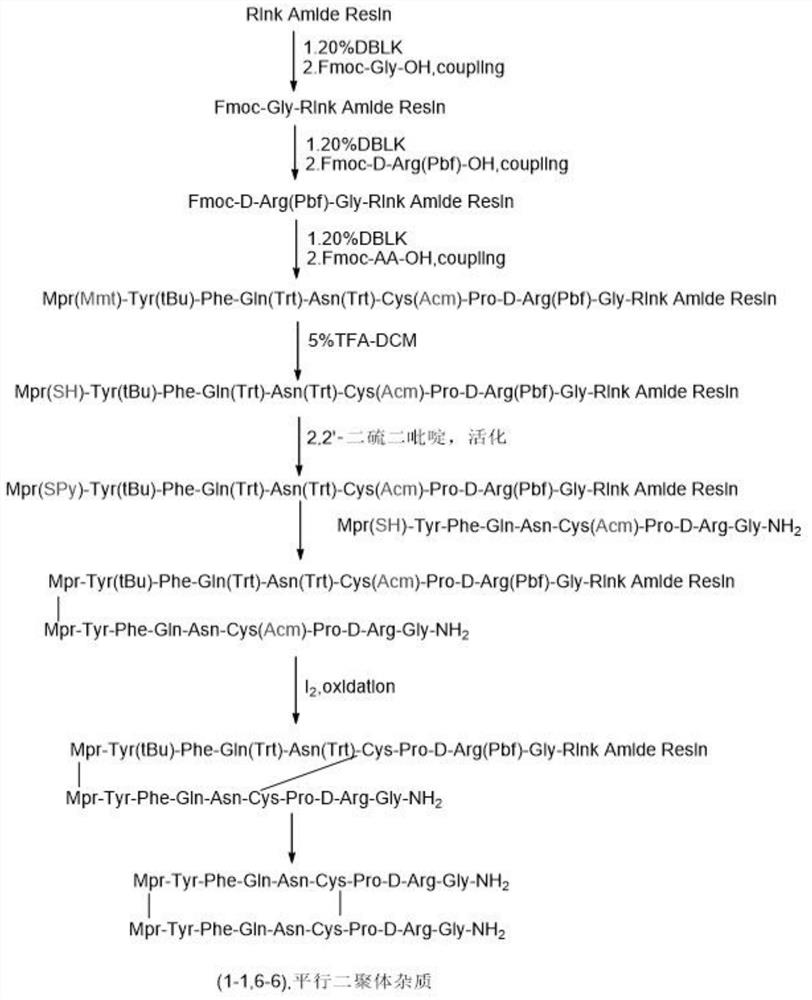
[0069] Such as figure 2 As shown, it is a synthetic route diagram of (1-1,6-6) dimer impurities in Example 2 of the present invention, and the specific synthetic route includes the following steps:
[0070] Peptide Resin Synthesis:
[0071] Weigh 11.9g of RinkResin resin (the degree of substitution is 0.42mmol / g, 5mmol), remove Fmoc and couple Fmoc-Gly-OH, Fmoc-D-Arg(Pbf)-OH, Fmoc in sequence according to the side chain of Fmoc solid phase synthesis -Pro-OH, FmocH-Cys(Acm)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Phe-OH, Fmoc-Tyr(tBu)-OH, Mpr(mmt )-OH. Finally, DMF was washed 3 times, DCM was washed 3 times, and methanol was shrunk to obtain 23.2 g of peptide resin. 16 g of the obtained peptide resin was cracked to prepare 1.0 g of linear peptide.
[0072] With 1-3 times the iodine, Acm is oxidized and removed to form another pair of disulfide bonds. Then use 10 times the TFA:TIS:H of the resin 2 Cleavage at O=95:3:2 to obtain (1-1, 1-6) dimer impurity peptide resin...
Embodiment 3
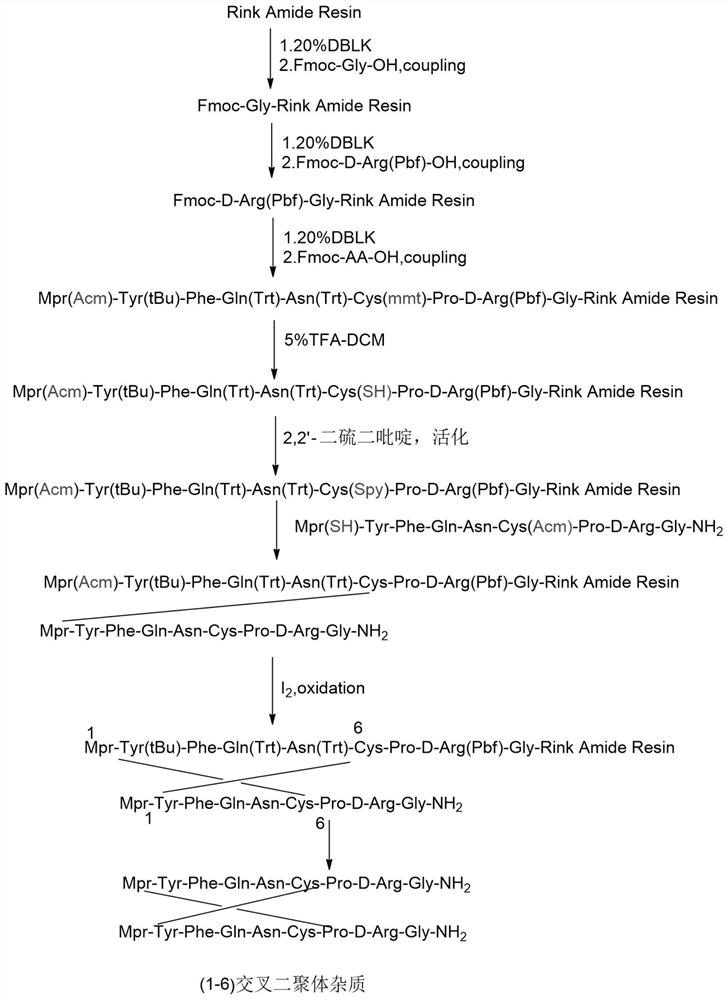
[0076] Such as image 3 As shown, it is a synthetic route diagram of (1-6) misplaced dimer impurities in Example 3 of the present invention, and the specific synthetic route includes the following steps:
[0077] Peptide Resin Synthesis:
[0078] Weigh 11.9g of RinkResin resin (the degree of substitution is 0.42mmol / g, 5mmol), remove Fmoc and couple Fmoc-Gly-OH, Fmoc-D-Arg(Pbf)-OH, Fmoc in sequence according to the side chain of Fmoc solid phase synthesis -Pro-OH, FmocH-Cys(mmt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Phe-OH, Fmoc-Tyr(tBu)-OH, Mpr(Acm )-OH. Finally, DMF was washed 3 times, DCM was washed 3 times, and methanol was shrunk to obtain 25.1 g of peptide resin. 18 g of the obtained peptide resin were cracked to prepare 1.3 g of linear peptides.
[0079] Synthesis of dimers:
[0080]Take 7g of peptide resin, add DCM to swell for 5 minutes, drain, then add DCM to swell for 5 minutes; repeat 2 times, drain, then add 120mL of 5% trifluoroacetic acid-DCM solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com