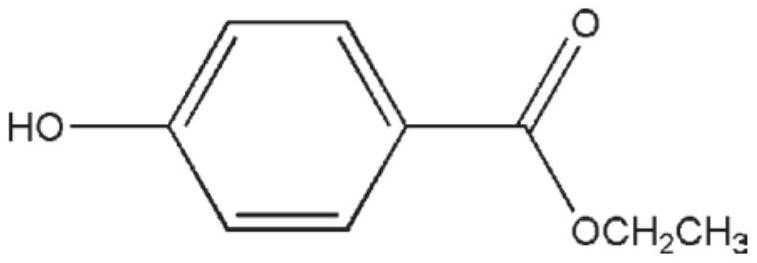
Application of ethyl p-hydroxybenzoate to resistance to coronavirus infection
A technology of ethyl hydroxybenzoate and coronavirus, which is applied in the field of medicine, can solve problems such as no reports and achieve good drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1 Butyl p-hydroxybenzoate atomized inhalation preparation
[0087] Butyl p-hydroxybenzoate 0.2g
[0089] Add water for injection to 1000ml
[0090] Preparation Process:
[0091] Step 1: Weigh 0.2g of butyl p-hydroxybenzoate and 9.0g of sodium chloride, add 1000ml of water for injection at 20°C to 35°C, dilute, stir evenly, and filter through a 0.22μm microporous membrane;
[0092] Step 2: Fill and seal in ampoules, and sterilize with high pressure steam at 115°C for 30 minutes.
Embodiment 2
[0093] Embodiment 2 Propyl p-hydroxybenzoate atomized inhalation preparation
[0094] Propylparaben 0.2g
[0096] Add water for injection to 1000ml
[0097] Preparation Process:
[0098] Step 1: Weigh 0.2 g of propyl p-hydroxybenzoate and 9.0 g of sodium chloride, add 1000 ml of water for injection at 20°C to 35°C, dilute, stir evenly, and filter through a 0.22 μm microporous membrane;
[0099] Step 2: Fill and seal in ampoules, and sterilize with high pressure steam at 115°C for 30 minutes.
Embodiment 3
[0100] Embodiment 3 Ethyl p-hydroxybenzoate atomized inhalation preparation
[0101] Ethyl p-hydroxybenzoate 0.2g
[0102] Sodium chloride 9.0g
[0103] Add water for injection to 1000ml
[0104] Preparation Process:
[0105] Step 1: Weigh 0.2g of ethyl p-hydroxybenzoate and 9.0g of sodium chloride, add 1000ml of water for injection at 20°C to 35°C, dilute, stir evenly, and filter through a 0.22μm microporous membrane;
[0106] Step 2: Fill and seal in glass ampoules, and sterilize with high-pressure steam at 115°C for 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com