Method for synthesizing straight-chain octadecanedioic acid
An octadecanedioic acid and straight-chain technology, applied in the field of synthesizing straight-chain octadecanedioic acid, can solve the problems of complex process, inability to industrialize production, and high cost of synthesizing straight-chain octadecanedioic acid, and achieve simple raw materials, Easy mass production, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a method for synthesizing linear octadecanedioic acid, comprising:
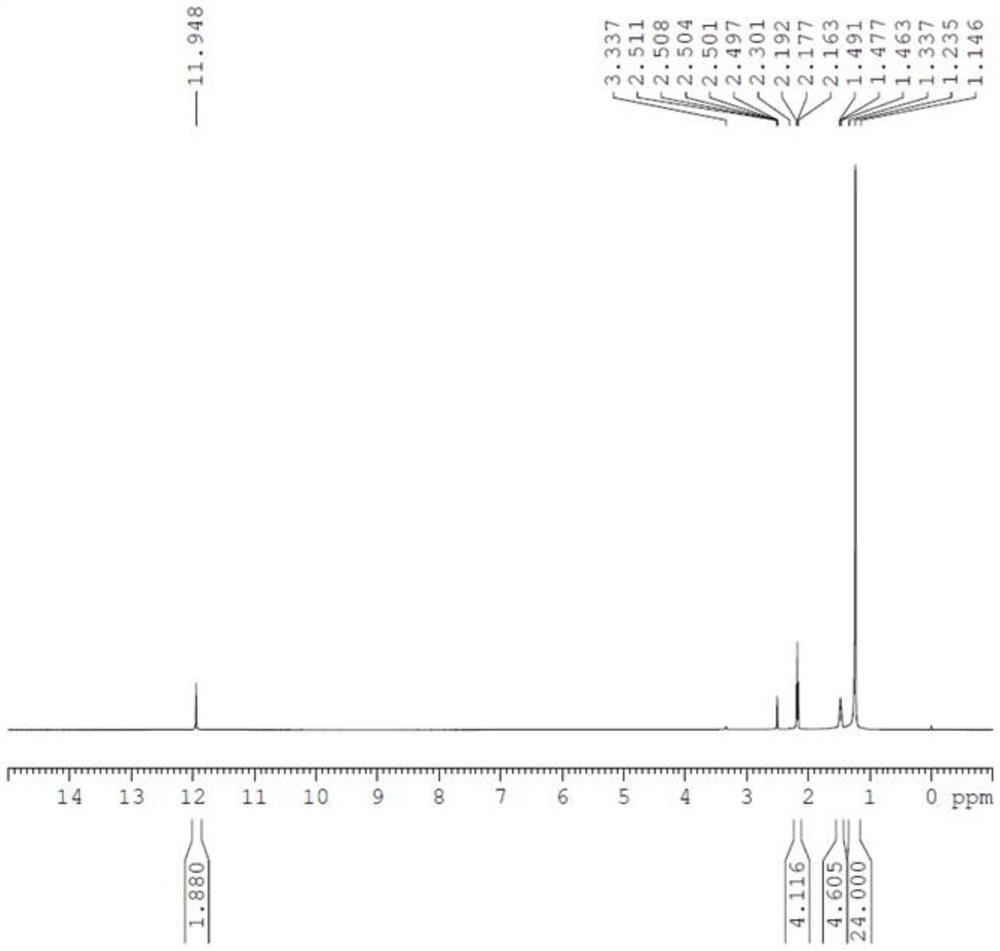
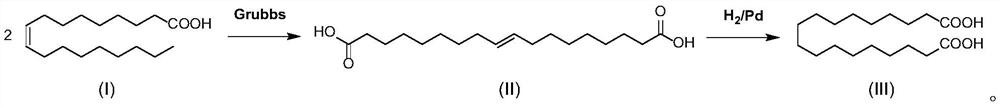
[0030] a) Under nitrogen protection, add 2340.0 kg of n-heptane, 600.0 kg of oleic acid, and 1.0 kg of Grubbs (II) catalyst into a 5000L glass-lined reactor, stir and heat up to 35-45°C. Insulated and reacted for 5 hours, then cooled to room temperature 20-25 ° C, centrifuged, the filter cake was washed with 160.0 kg of n-heptane, and the filter cake was dried to obtain 260.0 kg of the compound shown in formula II (9-octadecenedioic acid) , yield 78.4%;
[0031] b) Under nitrogen protection, add 3000.0 kg of tetrahydrofuran and 260.0 kg of the compound represented by formula II into a 5000L glass-lined reactor. After stirring to dissolve, the solution was transferred to a 5000L hydrogenation tank. 18.0 kg of 5% wet palladium carbon was added to the hydrogenation kettle, and after nitrogen replacement, hydrogen replacement was carried out to keep the pressure in the kettle at ...
Embodiment 2
[0033] This embodiment provides a method for synthesizing linear octadecanedioic acid, comprising:
[0034] a) Under nitrogen protection, add 1200.0 kg of n-heptane, 300.0 kg of oleic acid, and 0.6 kg of Grubbs (II) catalyst into a 3000L glass-lined reactor; stir and heat up to 40-50°C. Insulate and react for 4 hours, then cool down to room temperature 20-25°C, centrifuge, wash the filter cake with 160.0kg n-heptane, and dry the filter cake to obtain 140.0kg of the compound (9-octadecenedioic acid) shown in formula II , yield 84.4%.
[0035] b) Under nitrogen protection, add 2000.0 kg of tetrahydrofuran and 180.0 kg of the compound represented by formula II into a 5000L glass-lined reactor. After stirring and dissolving, transfer to a 5000L hydrogenation tank. Then add 15.0 kg of 5% wet palladium carbon to the hydrogenation kettle; after nitrogen replacement, hydrogen replacement is carried out to keep the pressure in the kettle at 0.3-0.6 bar, stir and raise the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com