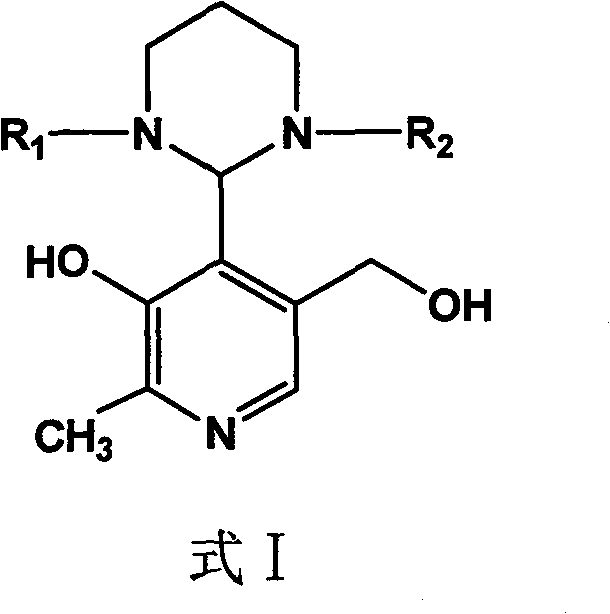
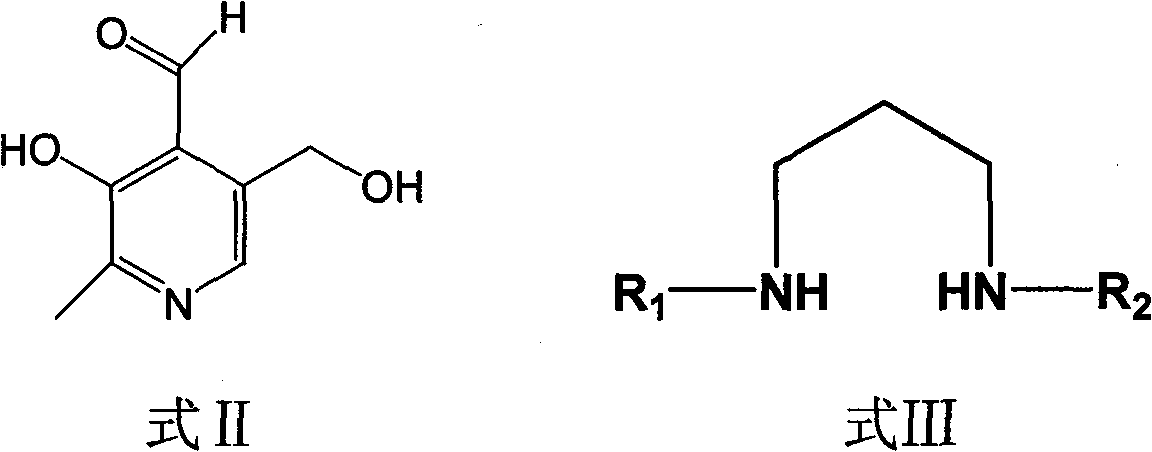
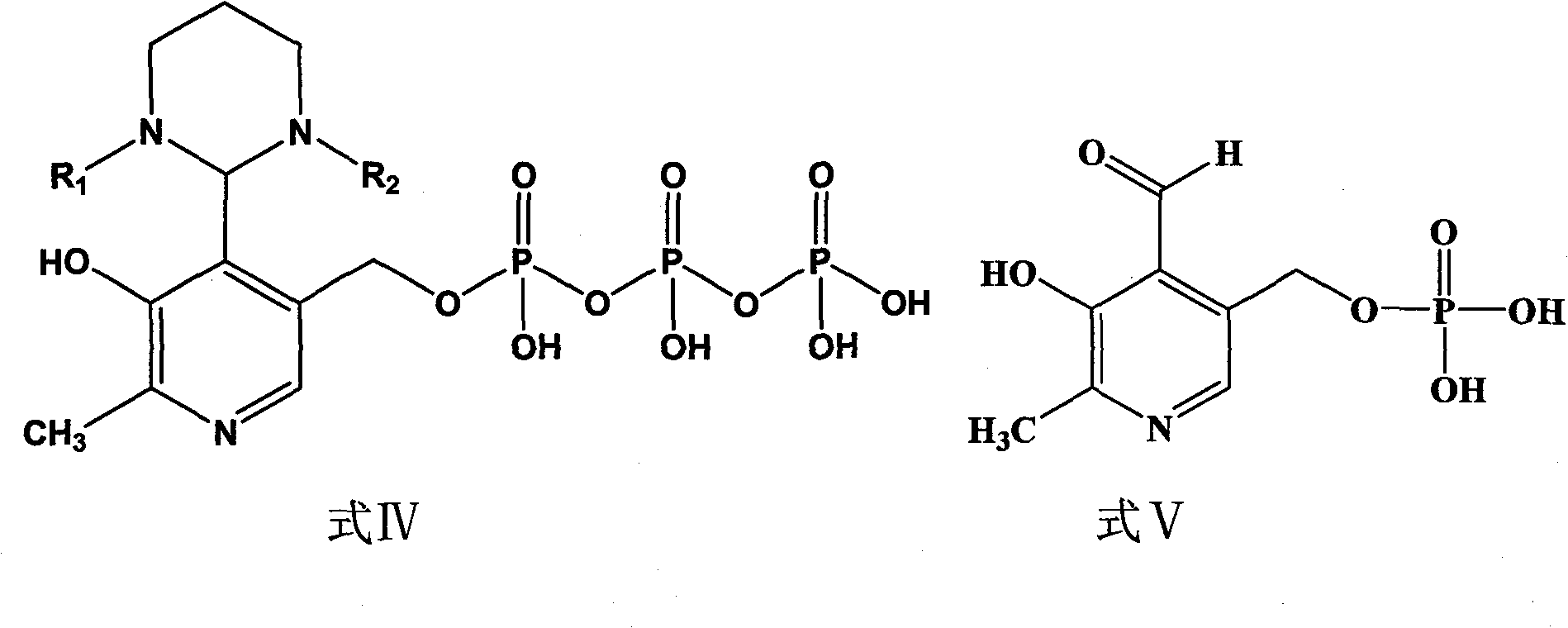
Phosphopyridoxal intermediates, preparation method of same, application of same, and method for preparing phosphopyridoxal therefrom
A technology of pyridoxal phosphate and intermediate, which is applied in the field of intermediate compound and preparation thereof, can solve the problems of difficult industrialized production, difficult source of raw materials, complicated steps and the like, and achieves the effects of simple operation, mild conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 N, the reaction of N'-diisopropylpropylenediamine and pyridoxal
[0035] In benzene (15ml), add pyridoxal (6.0mmol) and N, N'-diisopropylpropylenediamine (7.2mmol), heat up to 80°C for reflux and separate the produced water, continue the reaction until the thin layer Chromatography showed that the pyridoxal raw material disappeared, and the solvent was removed under reduced pressure, and ethyl acetate (10 ml) was recrystallized to obtain 1.39 g of the condensation product, with a yield of 75.5%.
[0036]
Embodiment 2
[0037] Embodiment 2 N, the reaction of N'-diethylpropylenediamine and pyridoxal
[0038] In toluene (15ml), add pyridoxal (6.0mmol) and N, N'-diethylpropylenediamine (9mmol), heat up to 110°C for reflux and separate the produced water, continue the reaction until thin layer chromatography It showed that the pyridoxal raw material disappeared, the solvent was removed under reduced pressure, and benzene (10 ml) was recrystallized to obtain 1.43 g of the condensation product, with a yield of 85.4%.
[0039]
Embodiment 3
[0040] Embodiment 3 N, the reaction of N'-dipropylpropylenediamine and pyridoxal
[0041] In xylene (20ml), add pyridoxal (6.0mmol) and N, N'-dipropylpropylenediamine (7.8mmol), heat up to 140°C for reflux and separate the produced water, continue the reaction until the thin layer Chromatography showed that the pyridoxal raw material disappeared, and the solvent was removed under reduced pressure, and the condensation product was recrystallized from toluene (15ml) to obtain 1.50g of the condensation product, with a yield of 81.4%.
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com