Gatifloxacin and its synthetic method
A technology of gatifloxacin and synthetic methods, applied in the direction of organic chemistry, etc., can solve problems such as time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention provides a synthetic method of gatifloxacin in one embodiment, comprising the steps of:
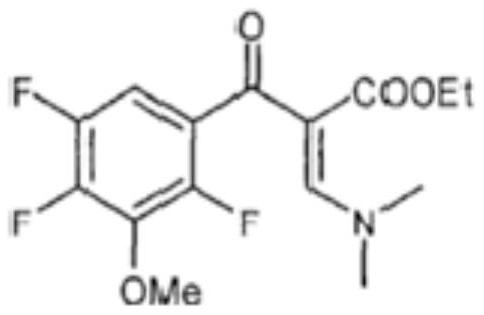
[0030] S1. Mix N,N-ethyl dimethylaminoacrylate, 2,4,5-trifluoro-3-methoxybenzoyl chloride, ethyl acetate and triethylamine, and obtain the first intermediate after the reaction is complete ; The structural formula of the first intermediate is as follows:
[0031]
[0032] The reaction principle of step S1 is that under the action of triethylamine, ethyl N,N-dimethylaminoacrylate is coupled with 2,4,5-trifluoro-3-methoxybenzoyl chloride in ethyl acetate The reaction affords the first intermediate.
[0033] In one embodiment, the ratio of N,N-ethyl dimethylaminoacrylate to 2,4,5-trifluoro-3-methoxybenzoyl chloride is (0.9˜1.1):1. The volume ratio of ethyl acetate to 2,4,5-trifluoro-3-methoxybenzoyl chloride is (10-20):1.
[0034] Further, the conditions for mixing and reacting N,N-ethyl dimethylaminoacrylate, 2,4,5-trifluoro-3-methoxybenzoyl chloride, ethy...
Embodiment 1
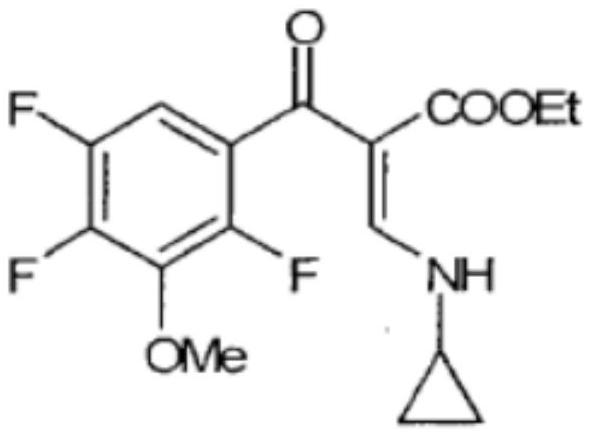
[0062] Put 15.0g of ethyl N,N-dimethylaminoacrylate into the reaction flask, then put in 11.11g of triethylamine and 100ml of ethyl acetate, stir and mix and heat to 60°C to get 2,4,5-trifluoro - 22.5g of 3-methoxybenzoyl chloride, dissolved in 100ml of ethyl acetate, then slowly drop the dissolved substance into the reaction flask, after 1 hour, the temperature was increased to 80°C, and the reaction was stirred for 2h. After cooling to room temperature, add 6.6 g of acetic acid for acidification, drop in 5.7 g of cyclopropylamine and continue stirring for 0.5 hour, add 120 ml of distilled water, transfer the mixed liquid to a separatory funnel, remove the upper water layer, and wash once with distilled water. Extract the aqueous layer with 100ml of ethyl acetate, combine the organic layers into the reaction flask, add 27.6g of potassium hydroxide, adjust the temperature to 140°C to remove water, cool after 7 hours, and distill the ethyl acetate under reduced pressure A brown...
Embodiment 2
[0066] Put 15.0g of ethyl N,N-dimethylaminoacrylate into the reaction flask, then put in 11.11g of triethylamine and 100ml of ethyl acetate, stir and mix and heat to 60°C to get 2,4,5-trifluoro - 22.5g of 3-methoxybenzoyl chloride, dissolved in 100ml of ethyl acetate, then slowly drop the dissolved substance into the reaction flask, after 1 hour, the temperature was increased to 80°C, and the reaction was stirred for 1h, After cooling to room temperature, add 6.6 g of acetic acid for acidification, drop in 5.7 g of cyclopropylamine and continue stirring for 0.5 hour, add 120 ml of distilled water, transfer the mixed liquid to a separatory funnel, remove the upper water layer, and wash once with distilled water. Extract the aqueous layer with 100ml of ethyl acetate, combine the organic layers into the reaction flask, add 27.6g of potassium hydroxide, adjust the temperature to 140°C to remove water, cool after 7 hours, and distill the ethyl acetate under reduced pressure A brown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com