Method for preparing 2, 4-disubstituted tetrahydropyran compound through hydrogenolysis reaction
A technology of tetrahydropyran compound and tetrahydropyran, applied in the direction of organic chemistry, etc., can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
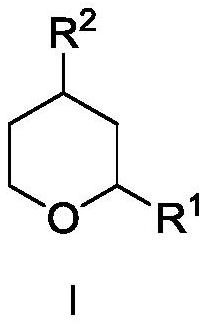
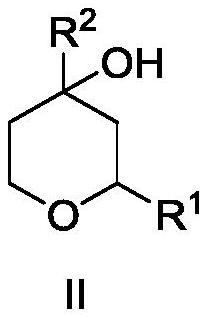
[0049] Synthesis of compound rose oxide I-1.
[0050] In a pressure-resistant reactor, add raw materials hydroxyrose ether II-1 (1mol, 1equiv, syn / anti is 3.56), hydrogen donor formic acid (5mol, 5equiv), organic phosphonic acid catalyst V-1 at room temperature (0.03mol, 3mol%) and 300mL of dichloromethane, keeping the stirring speed at 800rpm. The temperature program was started, and after the reaction temperature rose to 50° C., the reaction was continued for 4 h, and the reaction was stopped. The reaction was lowered to room temperature, the reaction kettle was opened, and the reaction solution was analyzed by gas-phase internal standard method. The conversion rate of hydroxyrose ether II-1 was 83%, the selectivity was 86%, and the product syn / anti was 3.57. Dichloromethane was recovered from the obtained oil phase under the conditions of 400hPaA and 25°C, and the raffinate was further purified by rectification under the conditions of 20 trays and a reflux ratio of 3 to o...
Embodiment 2
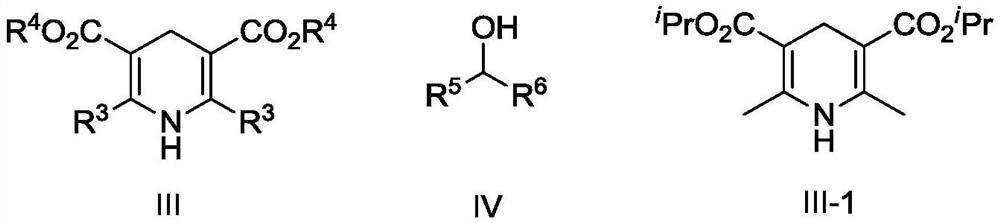
[0052] Synthesis of compound rose oxide I-1.
[0053] In a pressure-resistant reactor, add the raw material hydroxyrose ether II-1 (1mol, 1equiv, syn / anti is 3.56), hydrogen donor Hans ester III-1 in sequence at room temperature (5 mol, 5 equiv), organic phosphonic acid catalyst V-1 (0.03 mol, 3 mol%) and 300 mL of dichloromethane, and keep the stirring speed at 800 rpm. The temperature program was started, and after the reaction temperature rose to 50° C., the reaction was continued for 4 h, and the reaction was stopped. The reaction was lowered to room temperature, the reaction kettle was opened, and the reaction solution was analyzed by gas-phase internal standard method. The conversion rate of hydroxyrose ether II-1 was 97%, the selectivity was 98%, and the product syn / anti was 3.54.
Embodiment 3
[0055] Synthesis of compound rose oxide I-1.
[0056] In a pressure-resistant reactor, add the raw material hydroxyrose ether II-1 (1mol, 1equiv, syn / anti is 3.56), hydrogen donor Hans ester III-2 in sequence at room temperature (5 mol, 5 equiv), organic phosphonic acid catalyst V-1 (0.03 mol, 3 mol%) and 300 mL of dichloromethane, and keep the stirring speed at 800 rpm. The temperature program was started, and after the reaction temperature rose to 50° C., the reaction was continued for 4 h, and the reaction was stopped. The reaction was lowered to room temperature, the reaction kettle was opened, and the reaction solution was analyzed by gas-phase internal standard method. The conversion rate of hydroxyrose ether II-1 was 92%, the selectivity was 91%, and the product syn / anti was 3.55.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com