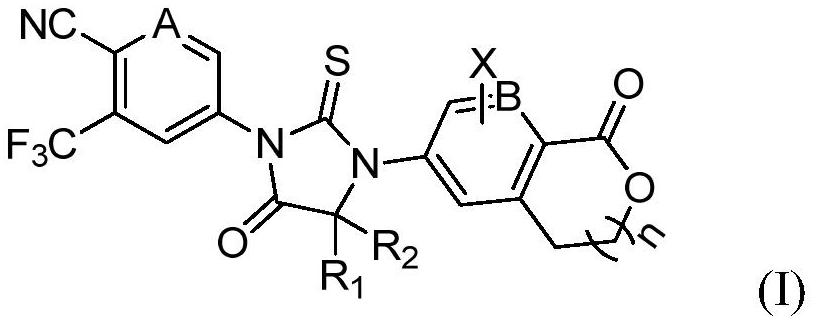
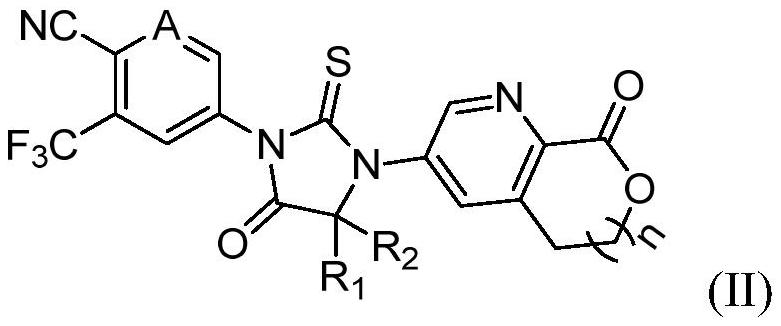
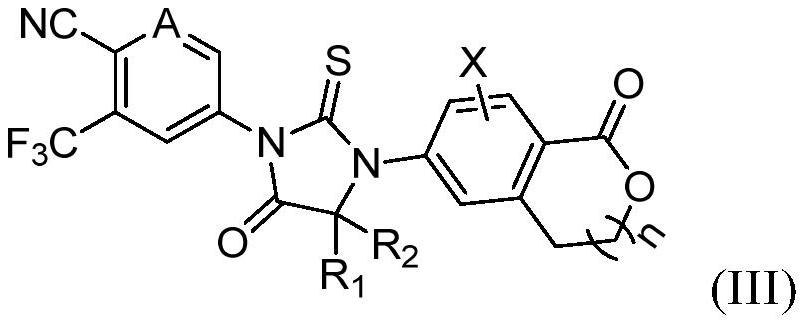
Aromatic ring and cyclic lactone thiohydantoin compound as well as preparation method and application thereof
A technology of compounds, hydrates, used in drug synthesis and pharmacology, the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: 4-(4,4-dimethyl-5-oxo-3-(1-oxo-1,3-dihydroisobenzofuran-5-yl)-2-thioimidazolidine -1-yl)-2-(trifluoromethyl)benzonitrile (compound 1)
[0074] 5-Aminoisobenzofuran-1(3H)-one (22)
[0075]
[0076] Copper sulfate (35mg, 0.22mmol, 0.007eq) was dissolved in 1.5mL of water, then zinc powder (5.5g, 84.11mmol, 2.8eq) was added to the solution, and then 12.75g of 20% sodium hydroxide solution was added. After stirring the reaction in the bath for several minutes, 5-aminoisoindoline-1,3-dione (5.00 g, 30.84 mmol, 1 equiv) was added. The reaction mixture was stirred at 60 °C for 4 h, TLC monitoring indicated the reaction was complete. The reaction was cooled to room temperature for suction filtration, and then the filter cake was washed with a small amount of water to obtain a filtrate, and then the pH was adjusted to 3-4 with concentrated hydrochloric acid. At this time, a large amount of solid precipitated out, and suction filtration was performed to obtain a y...
Embodiment 2
[0086] Example 2: 5-(4,4-Dimethyl-5-oxo-3-(1-oxo-1,3-dihydroisobenzofuran-5-yl)-2-thioimidazolidine -1-yl)-3-(trifluoromethyl)pyridinecarbonitrile (compound 2)
[0087] 5-isothiocyanato-3-(trifluoromethyl)pyridinecarbonitrile (25)
[0088]
[0089] Thiophosgene (0.489mL, 6.4mmol, 1.2eq) was dissolved in 20mL of water and stirred at room temperature, and 5-amino-3-(trifluoromethyl)cyanopyridine (1g, 5.37mmol, 1eq) was dissolved in batches added to the solution. The reaction mixture was stirred at room temperature for 4 h, TLC monitoring showed that the reaction was complete. Then the solution was extracted with dichloromethane, the organic phase was washed twice with saturated brine, washed with anhydrous Na 2 SO 4 After drying, filtration, the organic phase was concentrated by adding silica gel and purified by automatic column passer (0-10% ethyl acetate / petroleum ether) to obtain the desired product. Finally, 540 mg of light yellow oil was obtained, with a yield of 44...
Embodiment 3
[0093] Example 3: 4-(4,4-Dimethyl-5-oxo-3-(1-oxoisopyran-6-yl)-2-thioimidazolidin-1-yl)-2- (Trifluoromethyl)benzonitrile (Compound 3)
[0094] Methyl 4-amino-2-bromobenzoate (26)
[0095]
[0096] 2-Bromo-4-nitrobenzoic acid methyl ester (10g, 38.46mmol, 1 equivalent) was dissolved in 80mL ethanol and 20mL water, stirred at room temperature for several minutes, and then Fe (6.44g, 115.3mmol, 3 equivalents ) and NH 4 Cl (8.23g, 153.8mmol, 4eq) was added to the solution, and the temperature was slowly raised to 70°C. The reaction mixture was stirred at 70 °C for 5.5 h, TLC monitoring indicated the reaction was complete. The solution was then filtered with celite to obtain the filtrate, which was extracted with ethyl acetate, and the organic phase was washed twice with saturated brine, washed over anhydrous Na 2 SO 4 After drying, filtration and concentration, the desired product is obtained. Finally, 8.05 g of white solid was obtained with a yield of 90.99%. 1 H NMR (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com