Method for efficiently detecting anti-retina antibody in serum and application thereof
A technology for retinal and retinopathy, applied in the field of biomedicine, can solve the problems of a small number of detected ARAs, a single detection method, and a small sample content, and achieve the effects of reducing the blindness rate, high detection sensitivity, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
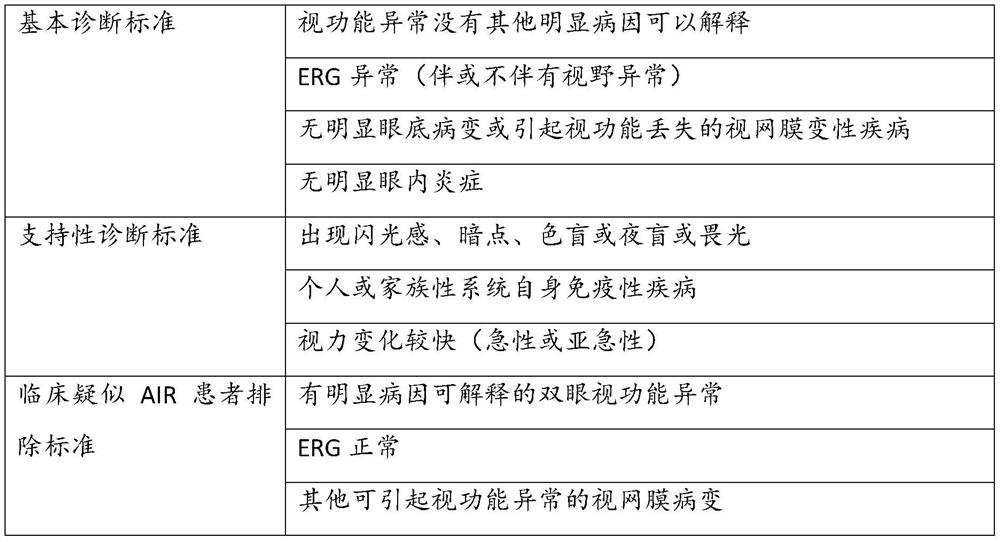
[0039] Clinical suspected AIR patients included Standards and Excluded Standards Reference 2016 US Veneulitis Association Published in AJO's Diagnosis and Treatment Consensus on Non-Vocal Taroid AIR (NP-AIR) (P-AIR patients with malignant tumors, other clinical The signs are similar to this, see Table 1).
[0040] Table 1
[0041]
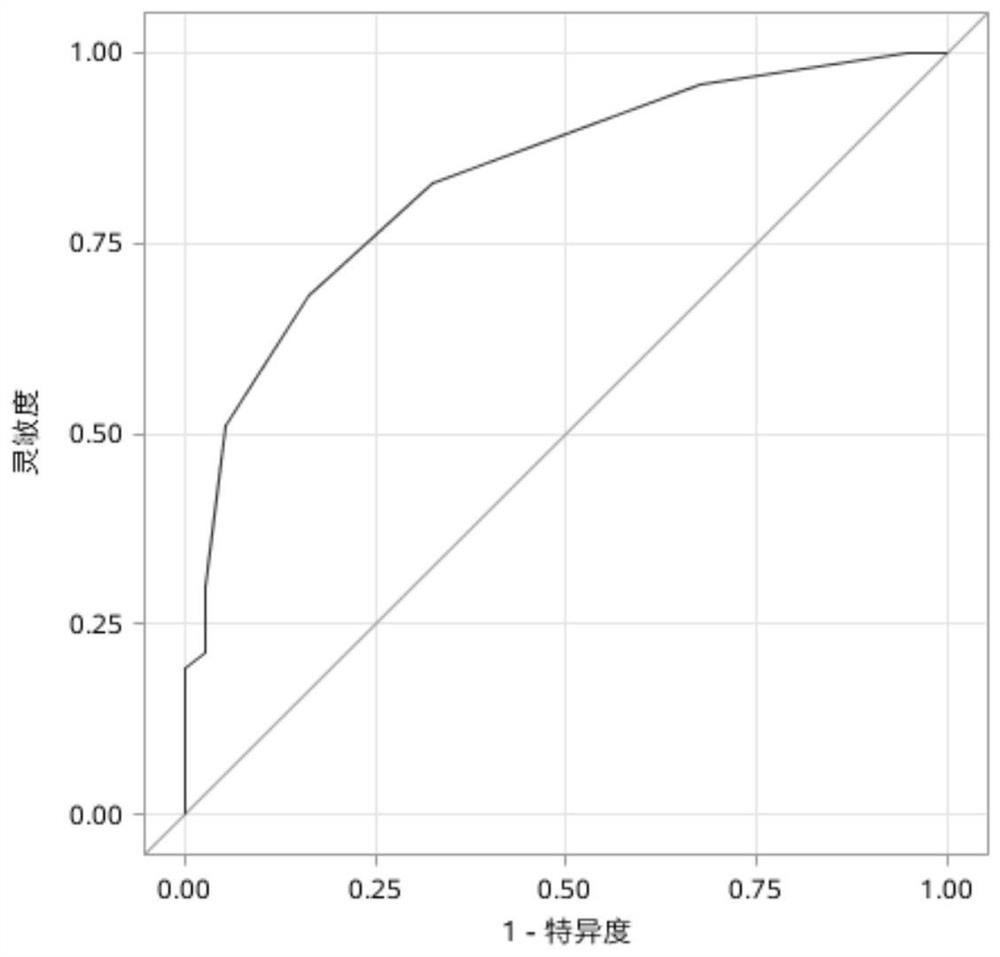
[0042] Experimental group: 58 patients with clinical suspected AIR, including 15 patients with P-AIR, 33 patients with NP-AIR.
[0043] Disease control group: 12 cases of patients with typical primary retinal pigmentation (RP) in patients with intraocular retransmitaria (RP) were selected according to the diagnosis of disease diagnosis.
[0044] Normal control group: No obvious retinal lesions were selected, 10 cases without obvious abnormal abnormalities.
[0045] Efficient detection of methods of ARAS in the serum of patients with AIR, including the following steps:
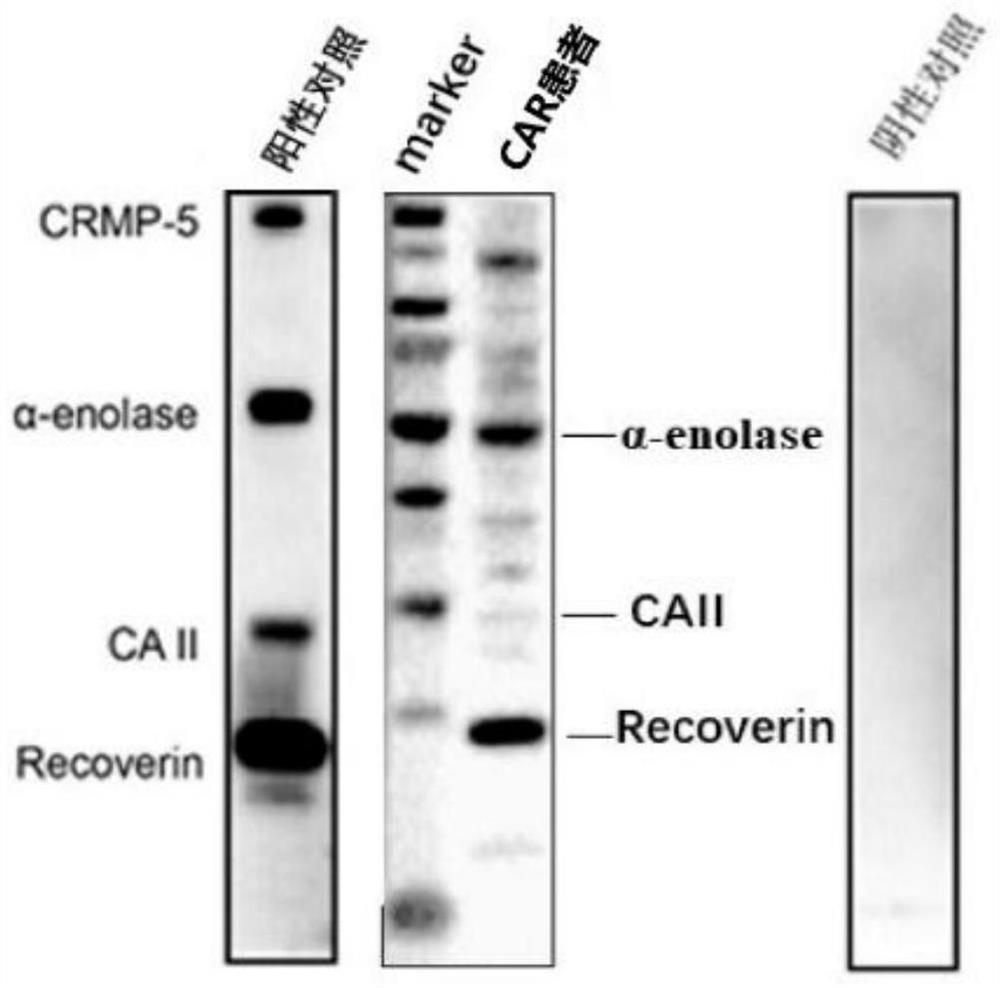
[0046] (1) Extract 5 ml of blood, place the extracted venous blood at 1500 rpm to...
Embodiment 2
[0071] The treatment programs were treated with 58 patients detected by Example 1 as follows:
[0072] Among the 15 P-Air patients, two newly diagnosed lung cancer patients received chemotherapy, and 13 P-Air patients had accepted the treatment of tumors (including resection of tumors, chemotherapy or radiotherapy). After testing, 12 patients were partially or / and whole body using glucocorticoids, and three patients chose to observe.
[0073] Among the 33 NP-AIR patients, 8 NP-AIR patients consolidated their own immune diseases. After testing, 26 patients started or continued to accept local or / and body sugar corticoids or cyclosporine treatment, 7 NP- AIR patient chose to observe.
[0074] Among the patients undergoing treatment, 30% (26 / 85) eyes were improved, 23% (16 / 85) patients were decreased, 53% (46 / 85) eyes were stable during the observation period.
[0075] Two types of retinal lesions have good response to Quannide (TA) or dexamethasone injection implants (ozurdex) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com