Preparation method of dimethyl adipate and lignin carbon-based solid acid catalyst
A technology of dimethyl adipate and solid acid catalyst, which is applied in the direction of carboxylic acid ester preparation, organic compound preparation, physical/chemical process catalyst, etc., and can solve the problem of long reaction time, high esterification temperature, There are many by-products of methyl esters, etc., to achieve the effect of reducing energy consumption and saving resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment introduces the amount of fixing adipic acid, methanol and reaction temperature, and by changing the amount of catalyst, the influence of the amount of catalyst on the conversion rate of adipic acid and the yield of dimethyl adipate is obtained:
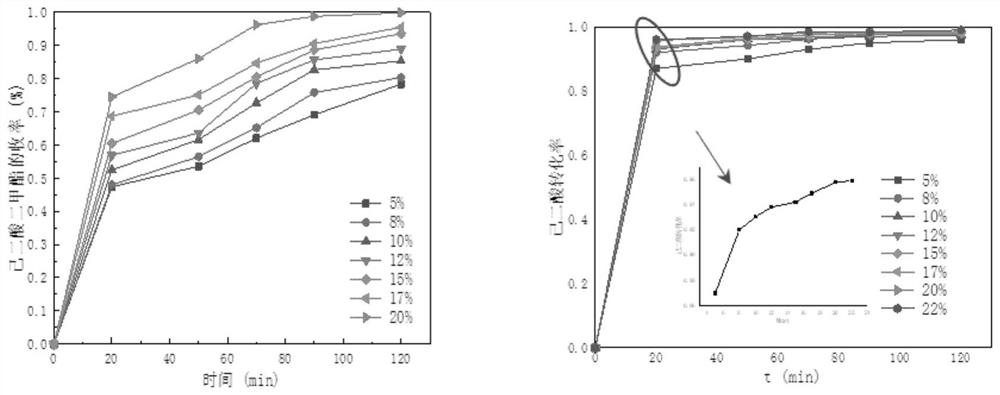
[0039] Such as figure 1 As mentioned above, in this embodiment, the amount of adipic acid is selected as 0.1 mol, the ratio of methanol to adipic acid is 7:1, the reaction temperature is 73°C, and the amount of catalyst is 5% of the mass of adipic acid. 8%, 10%, 12%, 15%, 17%, 20%, and 22% were used for batch catalysis experiments.
[0040] With the increase of the amount of catalyst, the catalytic effect is also gradually improved. Among them, the amount of catalyst has little influence on the conversion rate of adipic acid, which reaches about 90% in 50 minutes; while the effect on the yield of DMA is more obvious. The yield increased from 63.93% to 98.15% in 120 minutes. As the amount of catalyst added reac...
Embodiment 2
[0043] This example describes the effect of fixing the amount of adipic acid and constant reaction temperature, and changing the amount of methanol to obtain the influence of different acid-alcohol molar ratios on the yield of DMA and the conversion rate of adipic acid:
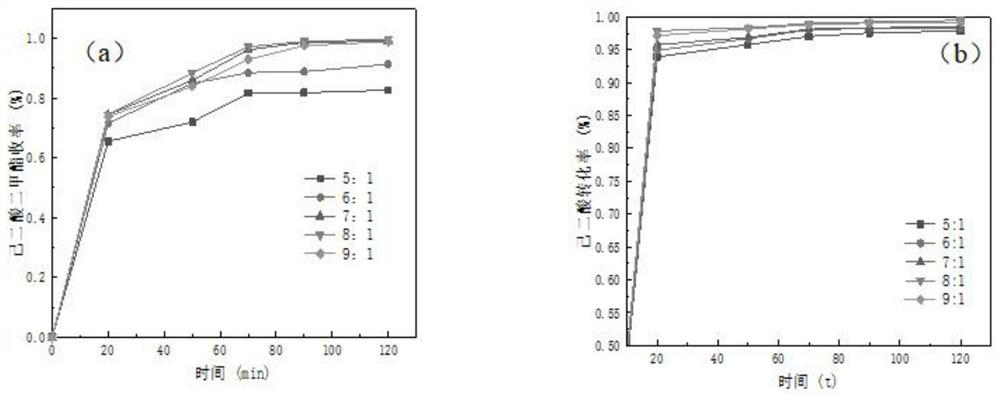
[0044] Such as figure 2 As shown, in order to maximize the conversion of the raw material adipic acid and to suppress the reverse reaction, an excess of methanol is selected. The amount of adipic acid is selected as 0.1mol as a benchmark, and when the reaction temperature is 73°C and the catalyst dosage is 20% of the mass of adipic acid, the alkyd-acid molar ratio is 4:1, 5:1, 6:1 , 7:1, 8:1, and 9:1 were used for catalytic experiments.
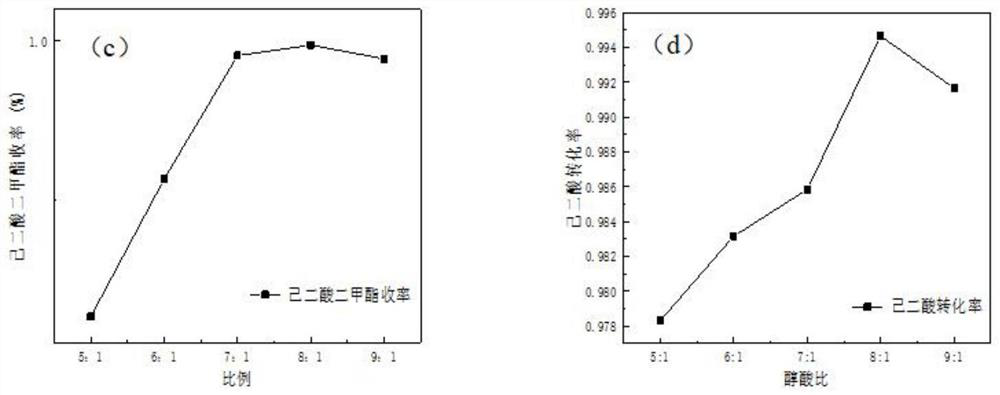
[0045] Experimental results such as figure 2 , image 3 shown, where figure 2 a and b show the change of DMA yield and adipic acid conversion rate with time under different alkyd ratios. Since the range of change is too small, the data at 120 minutes were taken for gr...
Embodiment 3
[0047] This embodiment has introduced the consumption of fixing adipic acid, methyl alcohol, catalyst, by changing reaction temperature, measures the impact of reaction temperature on the conversion rate of adipic acid and the yield of DMA:
[0048] Such as Figure 4 As shown, in order to determine the optimal temperature, the amount of catalyst is 20% of the mass of adipic acid, the molar ratio of alkyd to acid is 8:1, and the selected temperatures are 58°C, 63°C, 68°C, and 73°C for catalytic experiments. According to the experimental results, the conversion rate of adipic acid and the yield of DMA at the end of the reaction were selected as benchmarks.
[0049] From the experimental results, it can be concluded that as the temperature increases, the conversion rate of adipic acid and the yield of DMA both increase. In theory, the higher the temperature is, the more favorable the reaction is. At normal pressure, at 73°C, the bubble point temperature of the reaction has been ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com