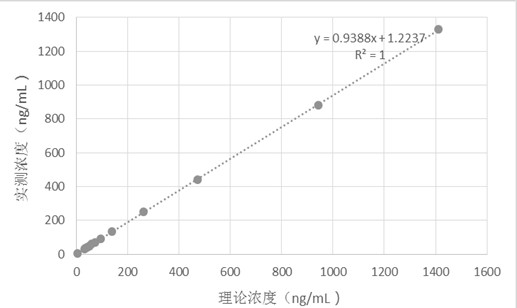
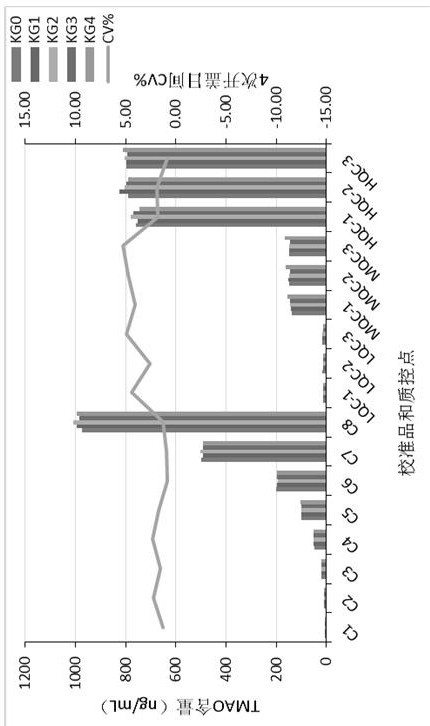
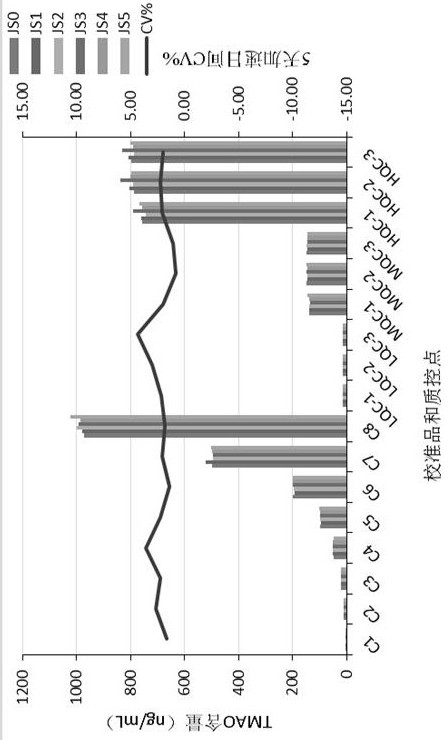
Kit for determining trimethylamine oxide as well as preparation method and application of kit
A trimethylamine oxide and kit technology, applied in the field of detection of endogenous biomarkers, can solve the inconsistency between batches of mixed matrix, the difficulty of applying in vitro diagnostic kits to industrialization, the source of mixed human blood matrix and ethical restrictions and other issues to achieve the effect of guaranteeing precision and accuracy and excellent acceleration stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation of the above kits provided by the present invention may include the following steps:
[0064] The known concentration of the trimethylamine oxide standard solution is diluted with a matrix solution to obtain the series of oxidized trimethylamine oxide calibrator working liquid having a concentration gradient; wherein the concentration of the known concentration of the trimethylamine oxide standard solution may, for example, be about 1mg / mL, subject to the actual weighing preparation; the known concentration of the oxidized trimethylamine standard solution may also be referred to as the trimethylamine oxide calibration reserve solution;
[0065] The deuterated trimethylamine standard solution of the known concentration of deuterium oxide trimethylamine is diluted with a matrix solution to obtain the predetermined concentration of the deuterated trimethylamine oxide standard working liquid; wherein the concentration of the known concentration of the deuterat...
Embodiment 1
[0093] 1.1. Prepare the kit as follows:
[0094] 1) Weighing: Weigh TMAO-d9 standards, BSA, TMAO standards respectively.
[0095] 2) Preparation of stock solution: TMAO standards were prepared with 50% (volume) aqueous methanol solutions into a concentration of 1mg / mL of trimethylamine oxide calibrator stock solution, a concentration of 1mg / mL of trimethylamine oxide quality control liquid, and TMAO-d9 standards were prepared with a 50% (volume) aqueous methanol solution with a concentration of 1mg / mL of deuterated oxidation trimethylamine reserve liquid.
[0096] 3) Matrix aqueous solution: The matrix BSA is formulated with 10mM pH 7.4 PBS to make a 5% matrix solution.
[0097] 4) The trimethylamine oxide calibrator reserve liquid, the oxidized trimethylamine quality control liquid, and the deuterium oxide trimethylamine internal standard reserve liquid are diluted with the matrix aqueous solution to obtain a series of trimethylamine oxide calibration working liquid with a concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com