Anti-IL-17A single-domain antibody and application thereof
A single-domain antibody, application-specific technology, applied in the direction of antibodies, resistance to vector-borne diseases, and anti-animal/human immunoglobulins, etc., can solve the problem of lack of anti-IL-17A single-domain antibody products, and achieve a wide range of affinity. , The effect of reducing production cost and simple transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Preparation of human IL-17A recombinant extracellular domain protein:
[0100] The human recombinant ectodomain protein used in this patent is obtained by the company's own expression and purification. The design scheme of the human IL-17A recombinant ectodomain protein expression vector is as follows:
[0101] (1) The coding sequence of IL-17A was retrieved from NCBI, its accession number is NM_002190.2, the amino acid sequence code generated by this sequence is NP_002181.1, and the Uniprot ID is Q16552.
[0102] (2) The amino acid sequence corresponding to NP_002181.1 was analyzed by TMHMM and SMART website respectively.
[0103] (3) The analysis results showed that IL-17A is a secreted protein, and positions 1-23 are the signal peptides of the protein.
[0104](4) The nucleotide sequence encoding amino acids 24-155 of IL-17A protein was cloned into the vector pcDNA3.4 by means of gene synthesis.
[0105] (5) Perform Sanger sequencing on the constructed v...
Embodiment 2
[0106] Example 2: Construction of single domain antibody library against IL-17A protein:
[0107] 1 mg of the recombinant human IL-17A protein purified in Example 1 was mixed with an equal volume of Freund's complete adjuvant to immunize an Inner Mongolia Alxa Bactrian camel once a week for a total of 7 consecutive immunizations, except for the first time. In addition to immunization, the other six times were immunized animals with equal volume of 1 mg IL-17A protein mixed with incomplete Freund's adjuvant.
[0108] After the animals were immunized, 150 mL of camel peripheral blood lymphocytes were extracted, and the RNA of the cells was extracted. cDNA was synthesized from the extracted total RNA, and VHH (variable region of antibody heavy chain) was amplified by nested PCR reaction using cDNA as template.
[0109] Then, the pMECS vector and the VHH fragment were digested with restriction endonucleases, respectively, and then the digested fragment and the vector were linked....
Embodiment 3
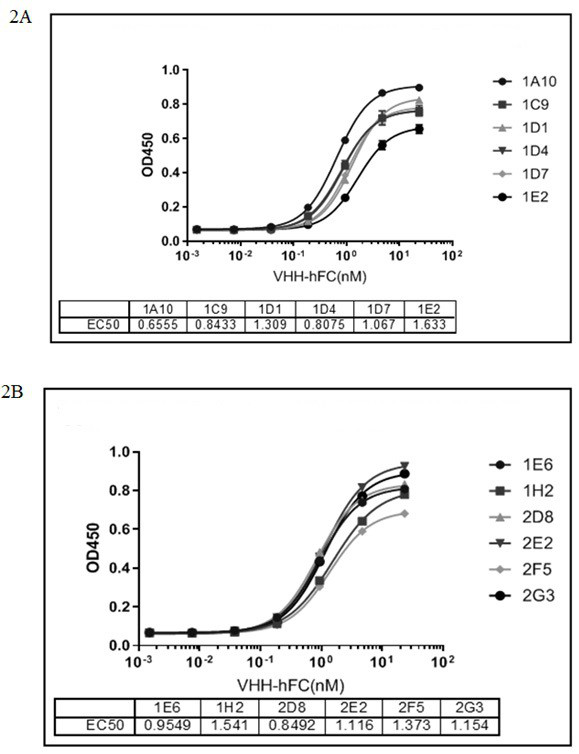
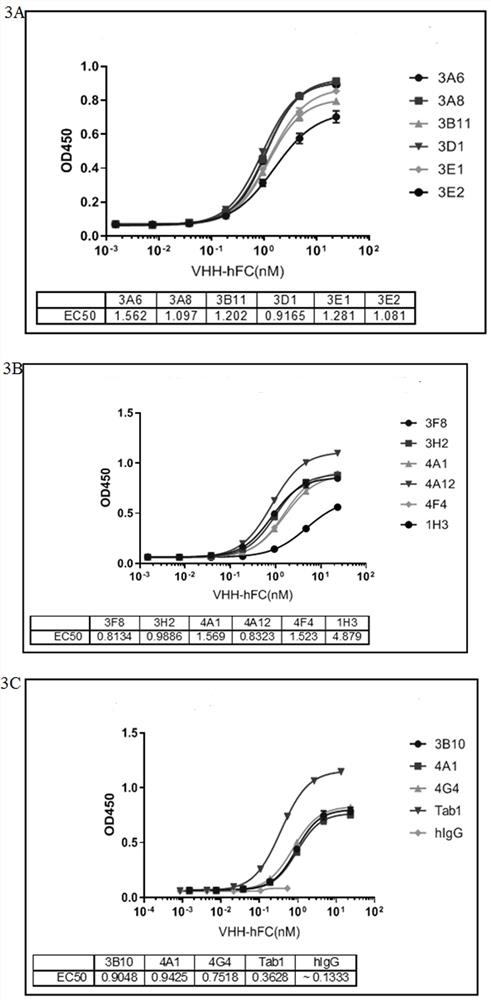
[0111] Example 3: Single domain antibody screening against IL-17A protein:
[0112] Take 200 μL of the recombinant TG1 cells in Example 2 and culture them in 2×TY medium. During this period, 40 μL of the helper phage VCSM13 was added to infect the TG1 cells, and the cells were cultured overnight to amplify the phages. The next day, the phages were precipitated with PEG / NaCl, and the expanded cells were collected by centrifugation. Bacteriophage.
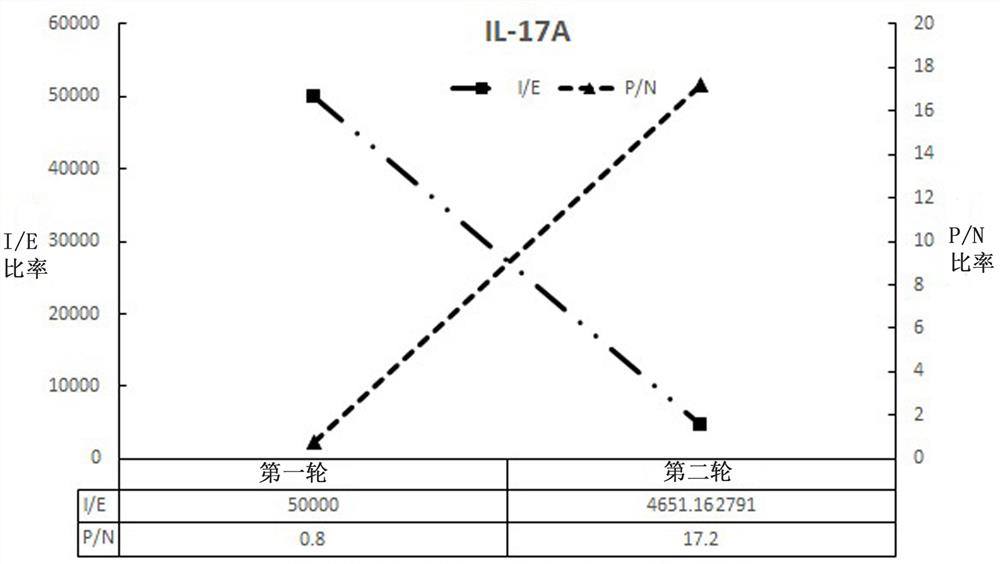
[0113] Dilute in 100 mM NaHCO pH 8.3 3 500 μg of IL-17A protein was coupled to the ELISA plate, placed at 4 °C overnight, and a negative control well (medium control) was set up; the next day, 200 μL of 3% skim milk was added, and it was blocked at room temperature for 2 hours; , add 100 μl of amplified phage library (approximately 2 × 10 11 phage particles) at room temperature for 1 h; after 1 h, wash 15 times with PBS+0.05% Tween-20 to wash off unbound phage.
[0114] The phage that specifically binds to the IL-17A protein was d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com