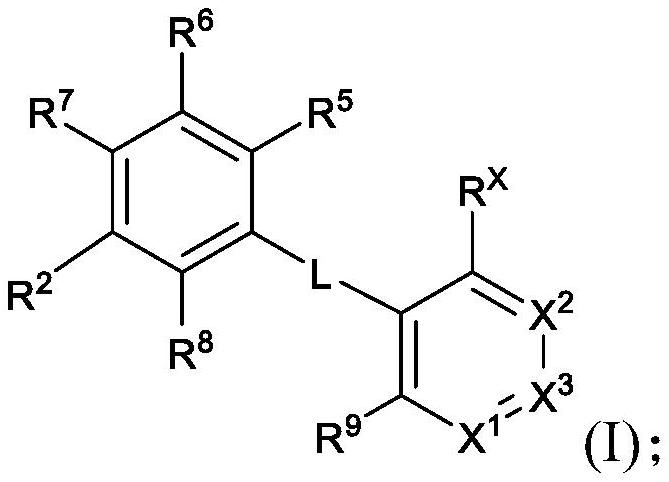
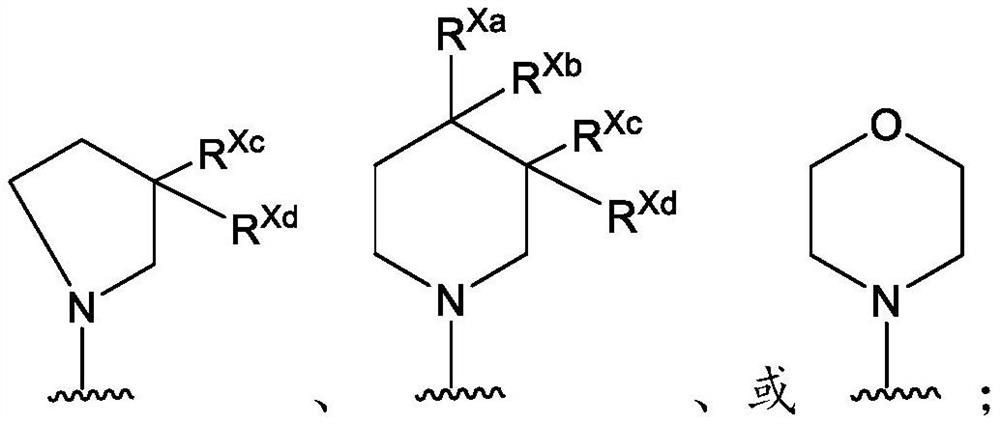
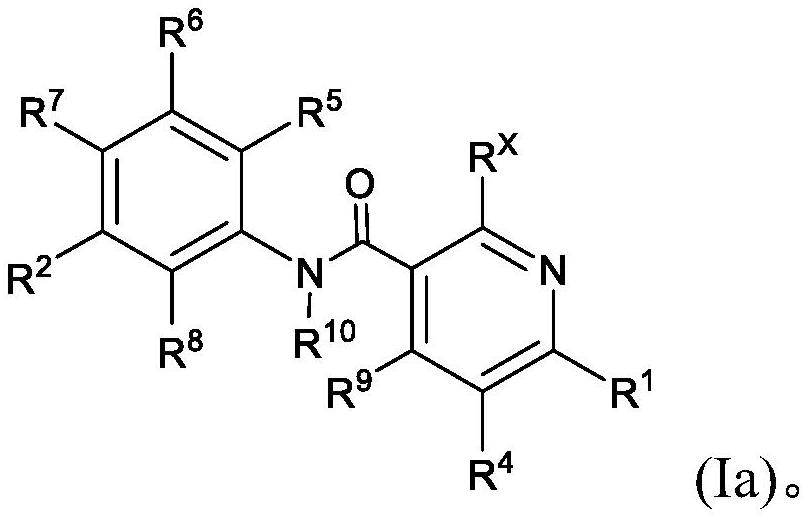
Pyridine derivatives as KIF18A inhibitors
The technology of a compound, -CR1, is applied in the field of KIF18A inhibitory activity, compounds and compositions that regulate KIF18A, manage cell proliferation and treat cancer, and prepare compounds of formula I
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0359] Ring Ar 1 Preparation of intermediates
[0360] Intermediate 1: 3-Amino-N-(tert-butyl)-5-methylbenzenesulfonamide.
[0361]
[0362] step 1: To ice-cold 1-methyl-3-nitrobenzene (2.0 g, 14.58 mmol) was slowly added chlorosulfonic acid (14.57 mL, 219 mmol) over 15 min. The resulting mixture was heated at 80 °C for 3 h. The reaction mixture was quenched with crushed ice and extracted with EtOAc (50 mL). The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to give 3-methyl-5-nitrobenzenesulfonyl chloride as a brown liquid. The crude sample was used immediately in the next step. 1 H NMR (400MHz, chloroform-d) δ8.70(t, J=1.9Hz, 1H), 8.52-8.39(m, 1H), 8.18(t, J=1.7Hz, 1H), and 2.66(s, 3H ).
[0363] step 2 : To an ice-cold solution of 2-methylpropan-2-amine (1.09 g, 14.94 mmol) and DIPEA (3.56 mL, 20.37 mmol) in DCM (50 mL) was slowly added 3-methyl-5-nitrobenzene- A solution o...
example 100
[0450] Example 100: 2-(4,4-Dimethylpiperidin-1-yl)-N-(3-(piperidin-1-ylsulfonyl)phenyl)nicotinamide.
[0451]
[0452] To a solution of 2-fluoro-N-(3-(piperidin-1-ylsulfonyl)phenyl)nicotinamide (44 mg, 0.12 mmol, Intermediate 14) in ACN (1 mL) was added 4,4-bis Methylpiperidine hydrochloride (33 mg, 0.22 mmol) and DIPEA (50 μL, 0.29 mmol). The reaction mixture was stirred at 85 °C for 4 h, then allowed to cool to RT. The reaction mixture was diluted with water (5 mL) and extracted with EtOAc (2x5 mL). The combined organic extracts were concentrated in vacuo and absorbed onto a plug of silica gel and chromatographed through a silica gel column (eluting with 0%-60% EtOAc in heptane) to provide 2-(4,4-di Methylpiperidin-1-yl)-N-(3-(piperidin-1-ylsulfonyl)phenyl)nicotinamide (35 mg, 0.08 mmol, 33% yield). 1 H NMR (chloroform-d) δ: 12.27 (brs, 1H), 8.42-8.61 (m, 2H), 8.14 (d, J=7.0Hz, 1H), 7.91-8.04 (m, 1H), 7.63 (d, J=5.9Hz,1H),7.48-7.57(m,2H),3.20(br s,4H),3.06(br s,4H),...
example 101
[0459] Example 101 : N-(3-(N-Cyclopropylsulfamoyl)phenyl)-2-(6-azaspiro[2.5]oct-6-yl)nicotinamide.
[0460]
[0461] 3-(2-(6-azaspiro[2.5]oct-6-yl)nicotinamido)benzene-1-sulfonyl chloride (0.049g, 0.12mmol, intermediate 15), cyclopropylamine (0.01g, 0.19 mmol) and DCM (1 mL) into a glass vial. To the reaction mixture was added DIPEA (0.065 mL, 0.37 mmol) and the mixture was stirred at rt for 2 h. The mixture was concentrated and the crude product was purified by silica gel chromatography (EtOAc in heptane 10%-70%) to afford N-(3-(N-cyclopropylsulfamoyl)phenyl) as a white solid - 2-(6-Azaspiro[2.5]oct-6-yl)nicotinamide (43 mg, 84% yield). 1H NMR (400MHz, chloroform-d) δ = 12.15 (br s, 1H), 8.50 (d, J = 6.3Hz, 2H), 8.39 (s, 1H), 7.94 (d, J = 8.0Hz, 1H), 7.69(d, J=7.8Hz, 1H), 7.61-7.53(m, 1H), 7.24(t, J=6.0Hz, 1H), 5.00(s, 1H), 3.28(t, J=5.3Hz, 4H ), 2.32 (d, J = 4.1 Hz, 1H), 1.63 (s, 4H), 0.75-0.59 (m, 4H), 0.43 (s, 4H). MS (ESI, cation) m / z: 427.1 [M+1].
[0462] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com