Camptothecin derivative and ligand-drug conjugate thereof
A technology of camptothecin and derivatives, applied in the field of camptothecin derivatives and their ligand-drug conjugates, which can solve problems such as toxic side effects, increased aggregation of ADC, and shortened half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0169] The preparation of conventional pharmaceutical compositions can be found in Chinese Pharmacopoeia.
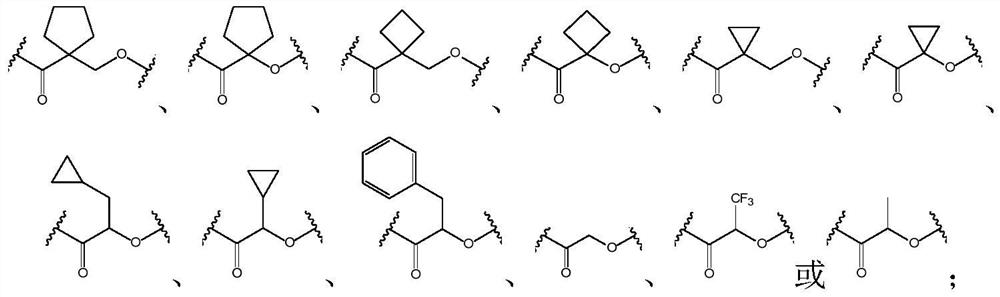
[0170] The term "pharmaceutically acceptable salt" or "pharmaceutically acceptable salt" refers to a salt of the ligand-drug conjugate of the present invention, or a salt of a compound described in the present invention, when such salt is used in a mammal It has safety and efficacy, and has proper biological activity. The ligand-drug conjugate compound of the present invention contains at least one carboxyl group, so it can form a salt with a base. Non-limiting examples of pharmaceutically acceptable salts include: sodium salt, potassium salt, calcium salt or magnesium salt, etc.
[0171] The term "pharmaceutically acceptable salt" or "pharmaceutically acceptable salt" refers to a salt of the antibody-drug conjugate of the present invention, or a salt of a compound described in the present invention, which has Safety and effectiveness, and have appropriate biological ac...
Embodiment 1
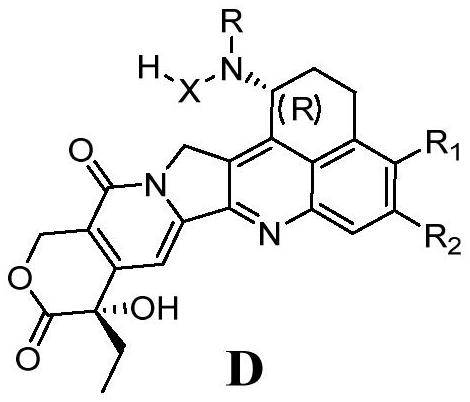
[0178] Embodiment 1: Synthesis of Exitecan Chiral Isomer Compound 1
[0179]
[0180] Weigh compound SM-1 (N-(8-amino-6-fluoro-5-methyl-1-oxo-1,2,3,4-tetrahydronaphthalene-2-yl ) acetamide, purchased) (6.25g, 25.0mmol), SM-2((S)-4-ethyl-4-hydroxyl-7,8-dihydro-1H-pyranO[3,4-F ] indolezin-3,6,10(4H)-one (purchased) (7.25g, 27.5mmol), pyridinium p-toluenesulfonate (1.26g, 5.0mmol), add 500mL toluene, heat to reflux to separate water, After reacting for 5 hours, the reaction was monitored by HPLC until SM-2 was less than 10%, and the reaction was stopped. Cool down, concentrate under reduced pressure until the volume of the solution is only half, stir and crystallize in an ice-water bath for 2 h, filter, wash the filter cake three times with 30 mL of toluene, and dry in vacuum to obtain compound Ac-1: 10.68 g, 89%. LC-MS gave [M+H] + :478.2.
[0181] Ac-1 (10.68g, 22.5mmol), methanesulfonic acid (55mL), water (110mL) and toluene (55mL) were mixed in a 1L round bottom flask ...
Embodiment 2
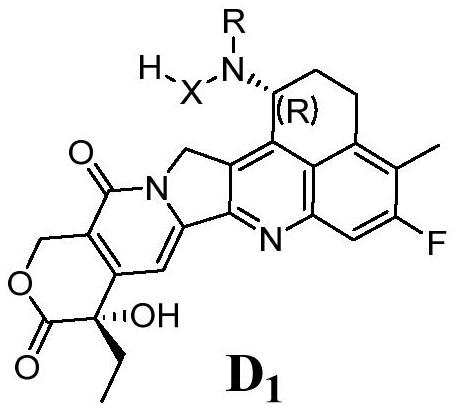
[0182] Embodiment 2: the synthesis of compound 2
[0183]
[0184] Add compound 1 (in the form of trifluoroacetate) (100.0mg, 0.188mmol), glycolic acid (43.0mg, 0.565mmol), HATU (107.2mg, 0.282mmol), HOBt (38.1mg, 0.282mmol) and 5ml ultra-dry DMF, with a liquid seal on the bottle. The flask was stirred under an ice-water bath for 10 minutes, then DIEA (94uL, 0.565mmol) was added. The progress of the reaction was detected by TLC, and the reaction was about 3 h. After the reaction was complete, the reaction solution was sent to the preparation for separation. The preparation solution was concentrated in vacuo at 35° C. and freeze-dried to obtain 63.8 mg of white solid compound 2 with a yield of 69.7%. LC-MS results show [M+H] + : 493.9. 1 H NMR (400MHz, DMSO-d6) δ8.39 (d, J = 9.0Hz, 1H), 7.68 (d, J = 10.9Hz, 1H), 7.29 (s, 1H), 6.51 (s, 1H), 5.53 (dt,J=9.1,6.0Hz,1H),5.41(s,2H),5.12(d,J=19.1Hz,1H),4.92(d,J=18.9Hz,1H),3.99(s,2H) ,3.10(qt,J=16.7,6.1Hz,2H),2.29(d,J=1.8Hz,3H)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap