Compositions and methods for treating hepatitis b virus infection
A hepatitis B virus and residue technology, applied in the direction of antiviral agents, virus antigen components, sugar derivatives, etc., can solve the problems of HbsAg loss and poor treatment tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
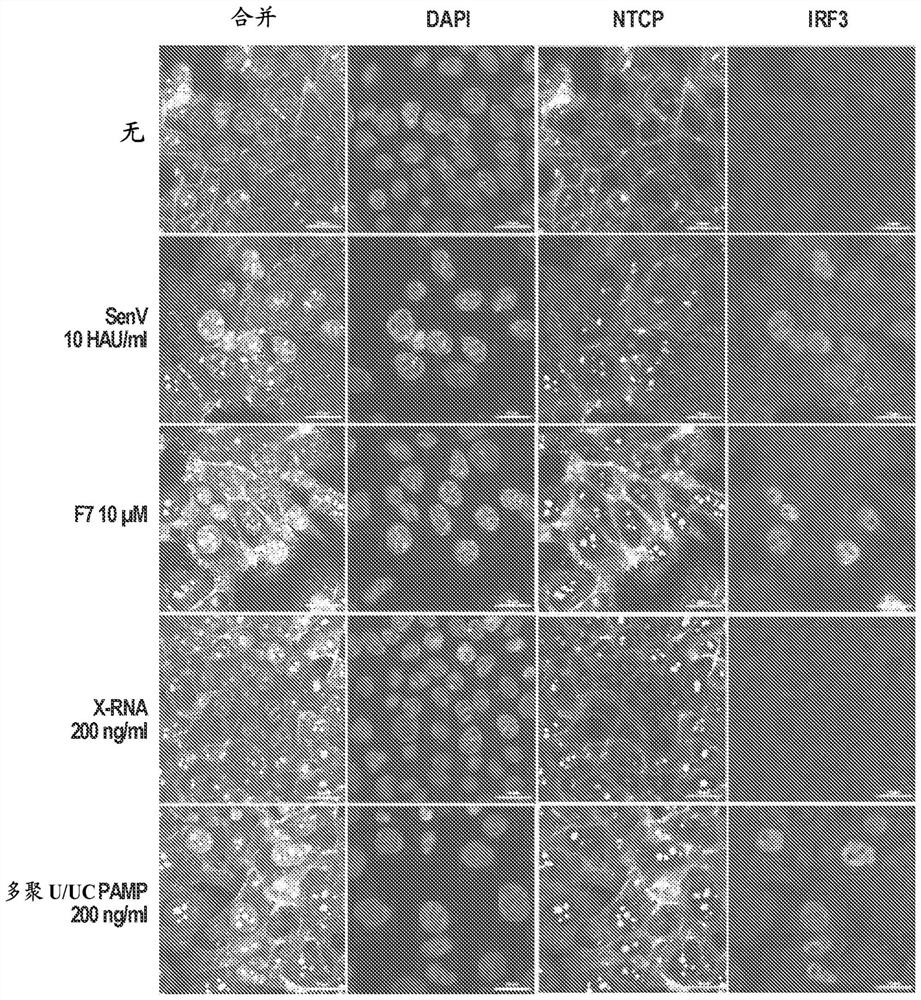
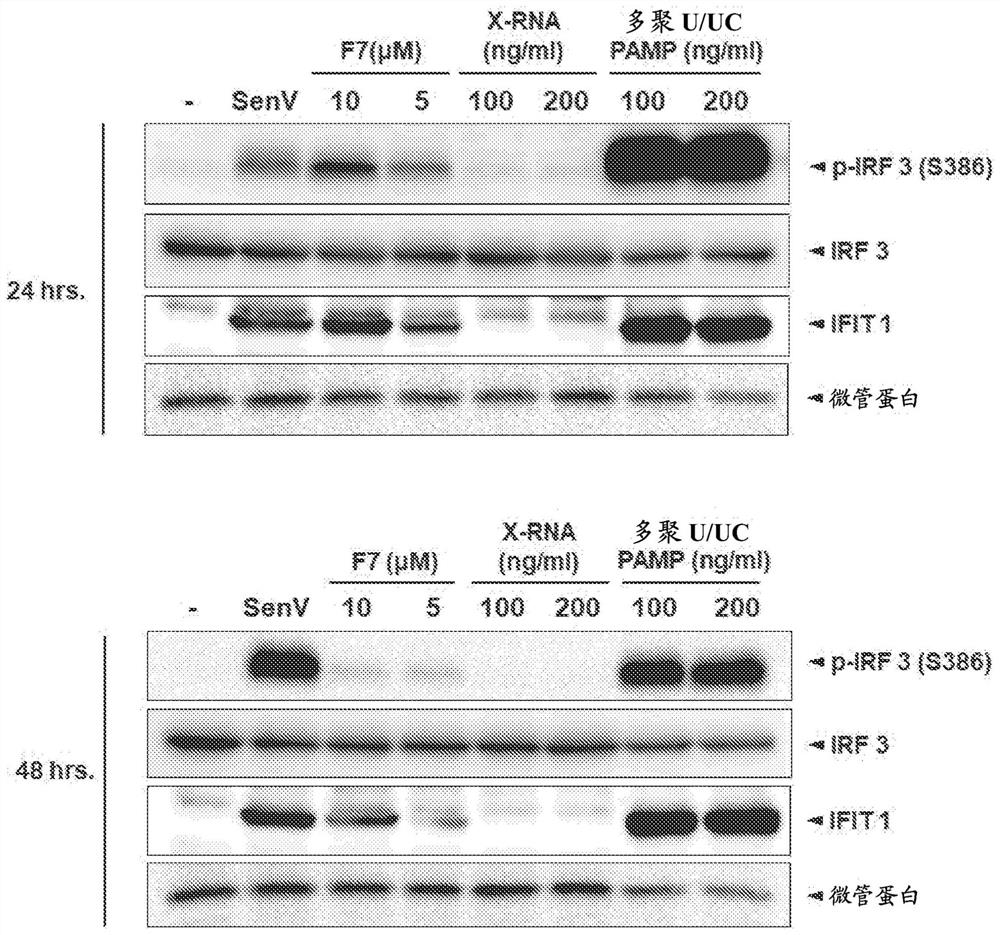
[0112] This example describes the demonstration that induction of the RIG-I signaling pathway destabilizes cccDNA and prevents the formation of new cccDNA in hepatocytes, providing a strategy for the eradication of cccDNA and corresponding hepatitis B virus infection.
[0113] introduction
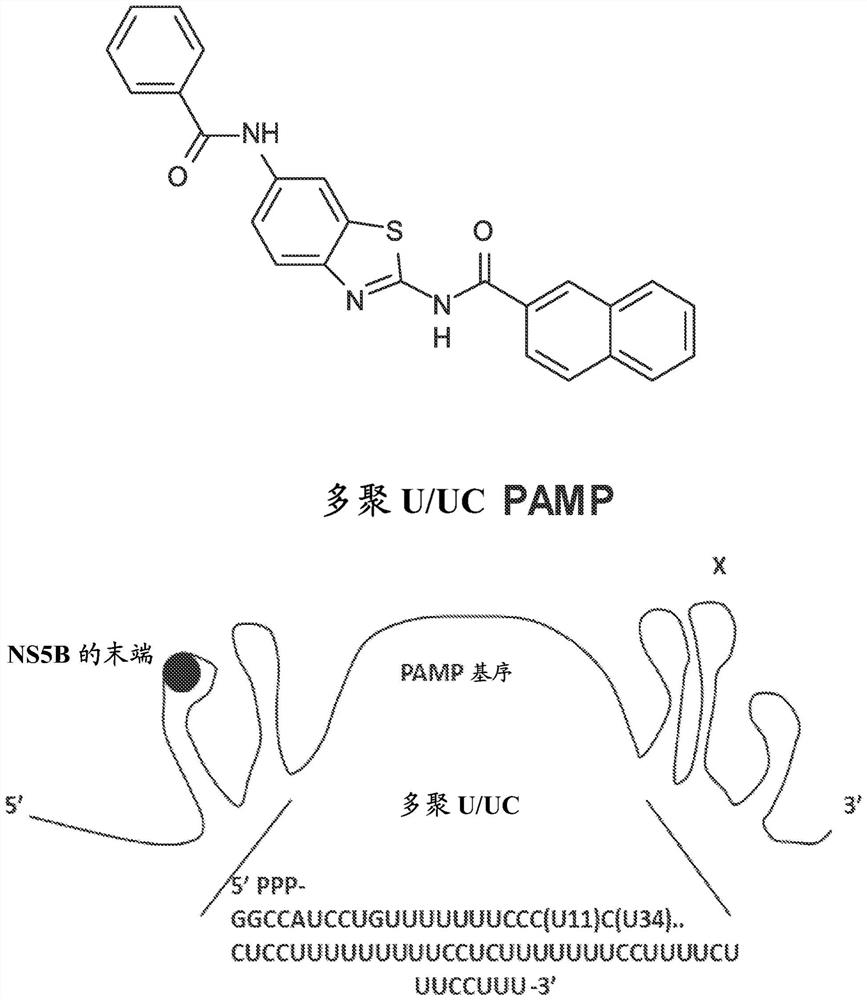
[0114] Acute viral infection often triggers the activation of intracellular innate immunity, leading to the induction of intracellular antiviral defenses. This process serves to control viral replication and spread from the site of infection, and to modulate the adaptive immune response for systemic viral control. Activation of innate immunity occurs through host cell perception of viral pathogen-associated molecular patterns (PAMPs) embedded in viral replication products, including viral nucleic acids. PAMPs are sensed by cellular pattern recognition receptors (PRRs). PRRs that sense viral infection include Toll-like receptors (TLRs), NOD-like receptors (NLRs), intracellular DNA senso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com