Synthesis method of quinoline compound intermediate
A synthesis method and compound technology, applied in organic chemistry, chemical recovery, etc., can solve the problems of low yield, complicated operation, unsuitable for industrial production, etc., achieve high yield, improve the purity of crude products, and be suitable for industrial scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
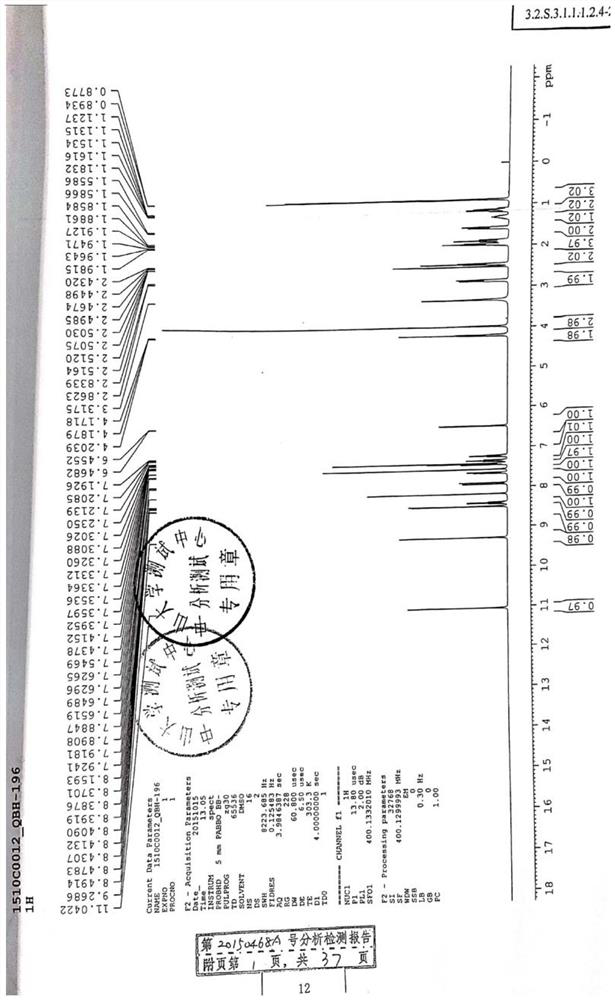
Image
Examples
Embodiment 1
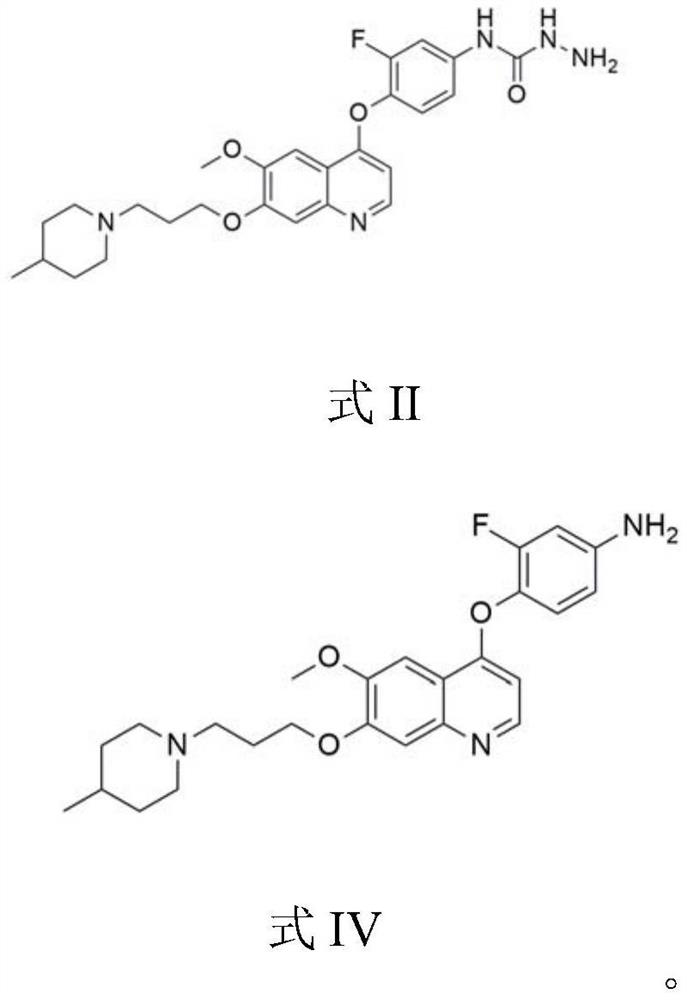
[0079] Example 1 Synthesis of the quinoline compound intermediate shown in formula II
[0080] The present embodiment provides a synthetic method of a quinoline compound intermediate shown in formula II, comprising the following steps:
[0081] 15.8kg (36mol, 1.0eq) of the compound of formula IV and 79L (5V) of NMP were added to the reactor, the temperature in the control reactor was 20~30°C, stirred for 0.75h until the reaction solution was dissolved, and 5.7kg (72mol, pyridine) was added. 2.0eq) into the reactor. Control the temperature in the reaction kettle to be 20~30°C, slowly add phenyl chloroformate 6.2kg (39.6mol, 1.1eq) dropwise to the reaction kettle, stir at 20~30°C for 2.5h, use HPLC to check that the compound of formula IV reacts completely ( 95%), add 15.7kg (252mol, 7.0eq) of 80% (w / w) hydrazine hydrate aqueous solution to the reaction kettle, and stir for 3h at 20-30°C. Slowly add 237L (15V) of purified water dropwise to the reaction kettle (dropping time 4....
Embodiment 2
[0097] Example 2 Synthesis of the quinoline compound intermediate shown in formula II
[0098] The present embodiment provides a synthetic method of a quinoline compound intermediate shown in formula II, comprising the following steps:
[0099] 15.8kg (36mol, 1.0eq) of the compound of formula IV and 79L (5V) of NMP were added to the reaction kettle, the temperature in the reaction kettle was controlled to be 20~30°C, stirred for 1h until the reaction solution was clear, and 5.7kg (72mol, 2.0%) of pyridine was added. eq) into the reactor. Control the temperature in the reaction kettle to be 20~30°C, slowly add 6.2kg (39.6mol, 1.1eq) of phenyl chloroformate dropwise to the reaction kettle, stir at 20~30°C for 2h, use HPLC to check that the compound of formula IV reacts completely (95%), add 15.7kg (252mol, 7.0eq) of 80% (w / w) hydrazine hydrate aqueous solution into the reaction kettle, and stir at 20-30°C for 2h. Slowly add 237L (15V) of purified water dropwise into the reactio...
Embodiment 3
[0100] Example 3 Synthesis of the quinoline compound intermediate shown in formula II
[0101] The present embodiment provides a synthetic method of a quinoline compound intermediate shown in formula II, comprising the following steps:
[0102] 15.8kg (36mol, 1.0eq) of the compound of formula IV and 79L (5V) of NMP were added to the reaction kettle, the temperature in the reaction kettle was controlled to be 20~30°C, stirred for 1h until the reaction solution was clear, and 5.7kg (72mol, 2.0%) of pyridine was added. eq) into the reactor. Control the temperature in the reaction kettle to be 20~30 ℃, slowly add phenyl chloroformate 6.2kg (39.6mol, 1.1eq) dropwise into the reaction kettle, stir at 20~30 ℃ for 6h, use HPLC to check that the compound of formula IV reacts completely (95%), add 15.7kg (252mol, 7.0eq) of 80% (w / w) hydrazine hydrate aqueous solution to the reaction kettle, and stir at 20-30°C for 6h. Slowly add 237L (15V) of purified water dropwise into the reaction k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com