Application of sanguinarine in preparation of RIPK3 activator
A sanguinarine, active technology, applied in the field of medicine, to achieve the effect of inhibiting activity, increasing the level of reactive oxygen species, and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
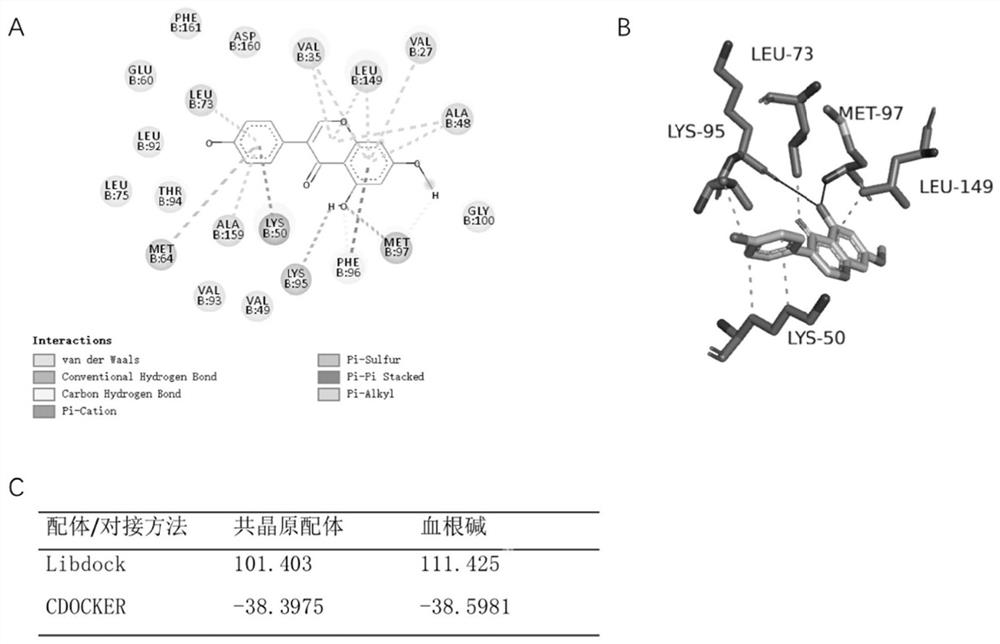
[0083] Example 1 Molecular docking
[0084] The RIPK3 protein crystal structure (PDB id: 7MX3) was obtained from the PDB database (https: / / www1.rcsb.org / ), and its structure was trimmed and hydrotreated by the "Prepare Protein" module of the Discovery Studio 4.5 platform, and removed at the same time. Water of crystallization, and the docking site is automatically identified based on the position of the original ligand in the PDB co-crystal structure. The "Prepare Ligand" module of the DiscoveryStudio platform was used to minimize the energy of sanguinarine through the CHARMM force field, and the Libdock and CDOCKER methods were used to run the docking. To form a control, we re-docked the PDB pro-co-crystal ligand into the protein structure, and compared the docking scores of the two to determine whether sanguinarine has better protein binding effect. Finally, the spatial interaction of the complexes was observed with the help of the visualization software Pymol.
[0085] Th...
Embodiment 2
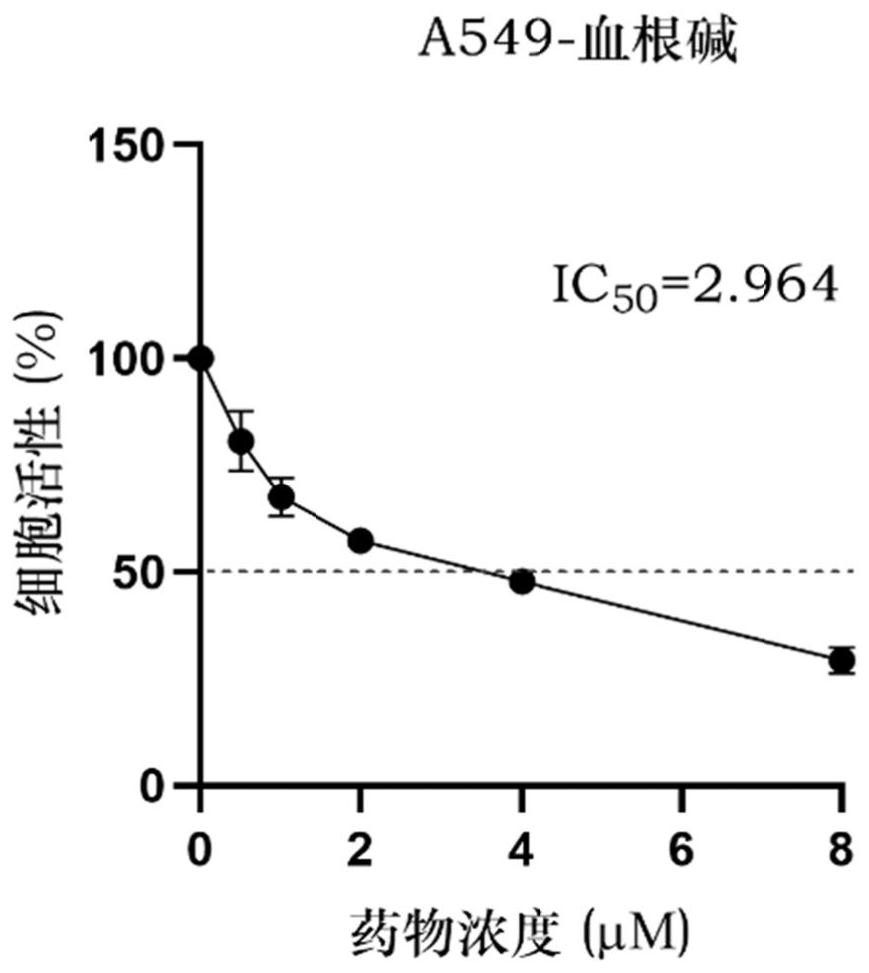
[0086] Example 2 The effect of sanguinarine on the activity of A549 cells
[0087] 1. Cell Culture
[0088] The well-grown A549 cells (cell line number: CCL-185) (all purchased from the American Culture Collection) were cultured, and the number of cells was 5 × 10 3 The density of cells / well was inoculated into a 96-well plate, and 100 μL of 1640 medium was added to each well, and placed in a constant temperature incubator (37°C, 5% CO). 2 ) for 24h. A549 cells were divided into control group (without any treatment, normal culture) and sanguinarine-treated group (final concentrations of sanguinarine were 0.5 μM, 1 μM, 2 μM, 4 μM, and 8 μM, respectively), and each treatment had 3 parallels, Continue to culture for 24h; use CCK-8 kit (Beyotime, catalog number: C0037) to detect the activity of A549 cells in 96-well plate, and use GraphPad prism software to calculate the IC of hemorrhizine acting on A549 cells 50 .
[0089] The result is as figure 2 As shown, the activity of...
Embodiment 3
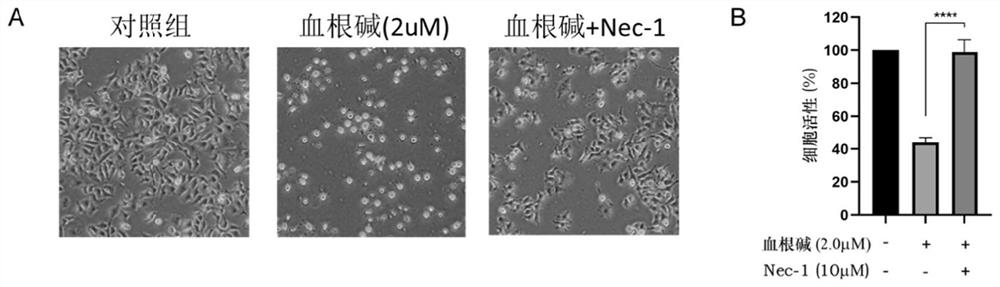
[0090] Example 3 The effect of sanguinarine on the proliferation of A549 cells
[0091] In order to explore how sanguinarine causes the death of A549 cells, A549 cells were analyzed for death in this example.
[0092] A549 cells in good growth state were seeded in 12-well plates, 10 × 10 per well. 4 100 μL of 1640 medium was added to each well, in 5% CO. 2 , cultured in a 37°C incubator for 24 h; cells were divided into control group (without any treatment, normal culture), sanguinarine treatment group (final concentration of sanguinarine was 2 μM) and sanguinarine and necroptosis inhibitory Co-treatment group (the final concentration of sanguinarine was 2 μM and the final concentration of Nec-1 was 10 μM) (Necrostatin-1, Nec-1), 3 parallels for each treatment; The state of cells in each group was observed under an electron microscope; at the same time, the activity of A549 cells in each treatment group was detected by using CCK-8 kit (Beyotime, product number: C0037).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com