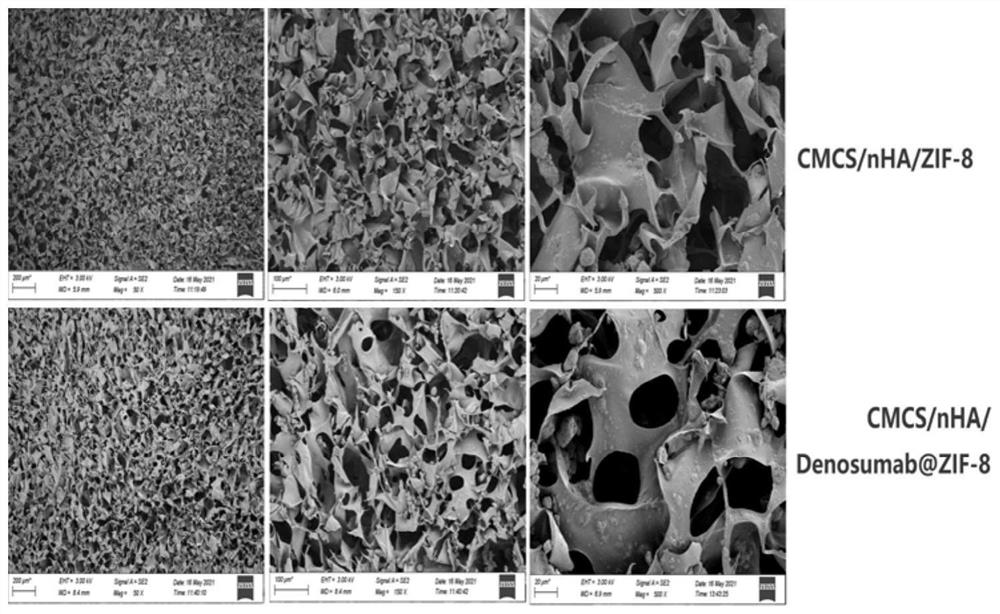
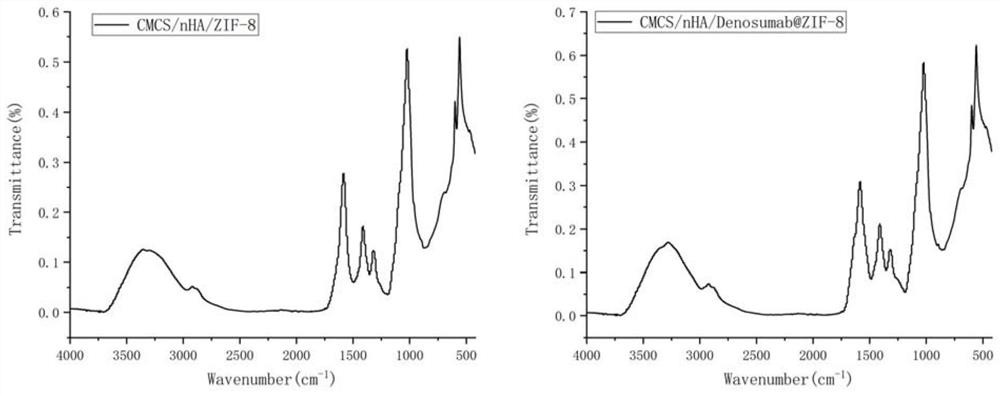
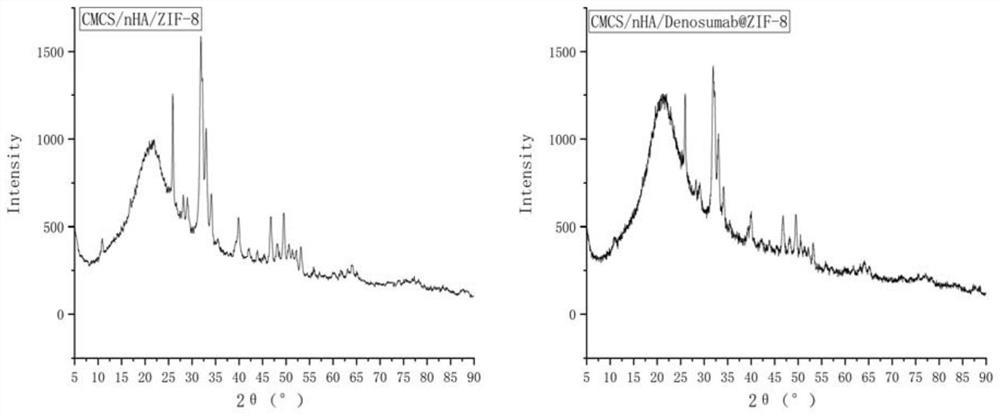
Desumab-loaded artificial bone and preparation method thereof
A technology of denosumab and artificial bone, applied in pharmaceutical formulations, prostheses, drug delivery, etc., can solve the problem of not being able to inhibit tumors, and achieve the effects of inhibiting bone resorption, inhibiting tumor cells, and improving bone quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Composite Gel Preparation
[0040] (1) Mixing: After adding Denosumab (120mg) to 100ml of deionized, then adding an appropriate amount of 0.1g ZIF-8 powder for physical mixing to obtain a Denosumab@ZIF-8 suspension, and then adding an appropriate amount of 2g carboxymethyl chitosan, After thorough physical mixing, the CMCS / Denosumab@ZIF-8 mixed gel was obtained, and then an appropriate amount of 4g nano-hydroxyapatite was added, and the CMCS / nHA / Denosumab@ZIF-8 mixed gel was obtained after thorough physical mixing;
[0041] (2) Stirring: use a constant temperature magnetic stirrer to stir evenly, stirring time is 30-60min, temperature is 20-30°C; the mass percentage of carboxymethyl chitosan in the suspension is 32.2%, and the mass percentage of nano-hydroxyapatite is 32.2%. The content was 64.3%, the mass percentage of denosumab was 1.9%, and the mass percentage of ZIF-8 was 1.6%.
[0042] 2. Freeze drying and forming
[0043] (1) Packing: the gel obtained in step...
Embodiment 2
[0051] 1. Composite Gel Preparation
[0052] (1) Mixing: Add Denosumab (360mg) to 100ml of deionized, and then add an appropriate amount of 0.1g 2-methylimidazole zinc salt (ZIF-8) for physical mixing to obtain Denosumab@ZIF-8 suspension, and then add an appropriate amount of 2g of carboxymethyl chitosan, fully physically mixed to obtain CMCS / Denosumab@ZIF-8 mixed gel, and then added an appropriate amount of 4g of nano-hydroxyapatite, and fully physically mixed to obtain CMCS / nHA / Denosumab@ZIF-8 mixed gel glue;
[0053] (2) Stirring: use a constant temperature magnetic stirrer to stir evenly, stirring time is 30-60min, temperature is 20-30°C; the mass percentage of carboxymethyl chitosan in the suspension is 31%, and the mass percentage of nano-hydroxyapatite is 31%. The content was 61.9%, the mass percentage of denosumab was 5.6%, and the mass percentage of ZIF-8 was 1.5%.
[0054] 2. Freeze drying and forming
[0055] (1) Packing: the gel obtained in step (1) is extracted...
Embodiment 3
[0063] 1. Composite Gel Preparation
[0064] (1) Mixing: Add Denosumab (720mg) to 100ml of deionized, and then add an appropriate amount of 0.1g 2-methylimidazole zinc salt (ZIF-8) for physical mixing to obtain Denosumab@ZIF-8 suspension, and then add an appropriate amount of 2g of carboxymethyl chitosan, fully physically mixed to obtain CMCS / Denosumab@ZIF-8 mixed gel, and then added an appropriate amount of 4g of nano-hydroxyapatite, and fully physically mixed to obtain CMCS / nHA / Denosumab@ZIF-8 mixed gel glue;
[0065] (2) Stirring: use a constant temperature magnetic stirrer to stir evenly, stirring time is 30-60min, temperature is 20-30°C; the mass percentage of carboxymethyl chitosan in the suspension is 29.3%, and the mass percentage of nano-hydroxyapatite is 29.3%. The content is 58.7%, the mass percentage content of denosumab is 10.5%, and the mass percentage content of ZIF-8 is 1.5%.
[0066] 2. Freeze drying and forming
[0067] (1) Packing: the gel obtained in ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com