Chimeric antigen receptor capable of enhancing anti-tumor capability of CAR-T cell, D-CAR-T cell and application of D-CAR-T cell
A D-CAR-T, chimeric antigen receptor technology, applied in the field of cell therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Zeta-CAR and D-CAR plasmids were successfully constructed using molecular cloning technology, and the specific experimental conditions are as follows.
[0046] Construction and identification of 1Zeta / D-CAR plasmid
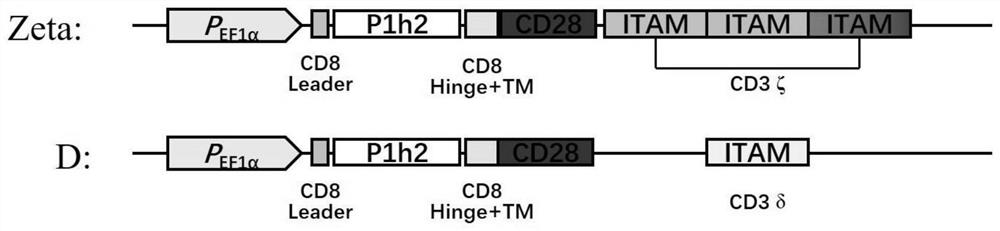
[0047] Based on the classic second-generation CD28-CD3ζ-CAR structure, the molecular cloning technology was used to change the CD3ζ molecule containing 3 ITAM motifs into a δ sequence containing only 1 ITAM, and prepared CAR structures with different intracellular signal intensities ( figure 1 ).
[0048] 1.1 Primer design and PCR
[0049] (1) According to the base sequences of Zeta-CAR and D-CAR, Qingke Xinye Biotechnology (Beijing) Co., Ltd. carried out whole gene synthesis, and used this as a template to amplify Zeta respectively using the primers shown in Table 1. -CAR and D-CAR genes:
[0050] Table 1 Amplification Primers
[0051] name Primer sequence (5'-3') Zeta-F GGTACGAATTCGCCACCATGGCACTGCCCGTC (SEQ ID NO: 5) Zeta-R ...
Embodiment 2
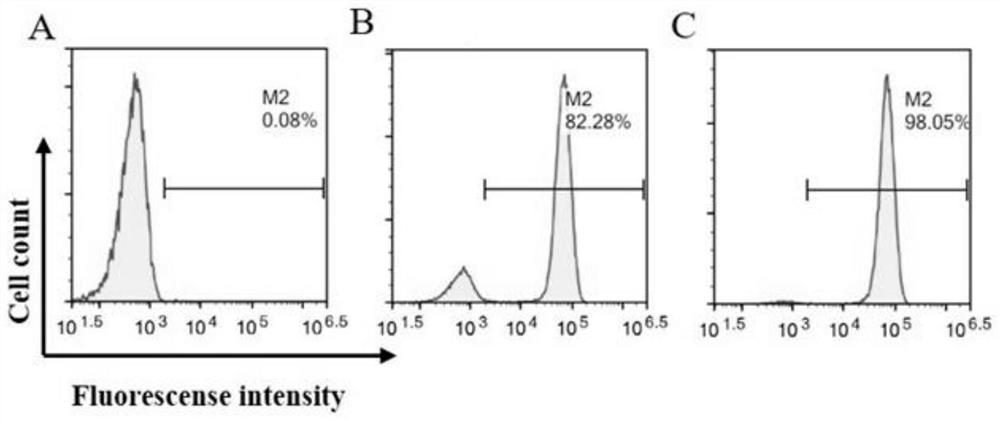
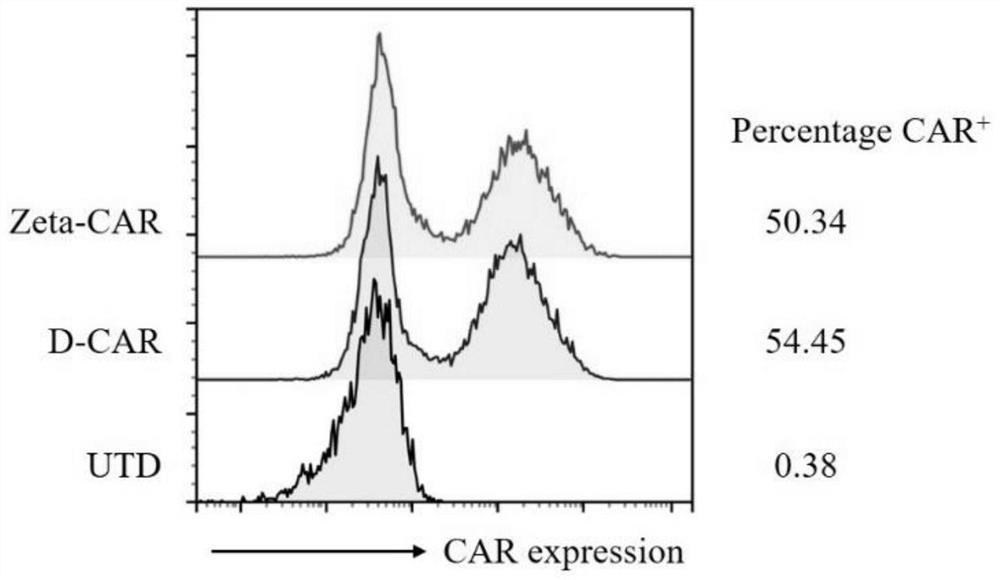
[0082] 1 Preparation of CAR-T cells
[0083] 1.1 Cell recovery and subculture
[0084] (1) Take HEK-293T cells out of liquid nitrogen and place them in a 37°C water bath to melt quickly, transfer the cell suspension into a 15mL centrifuge tube in a sterile ultra-clean workbench, add 4-5 times the volume of 10 % fetal bovine serum DMEM complete culture solution, centrifuged at 800 rpm for 5 min, discarded the supernatant, and repeated this step twice to obtain cell pellets.
[0085] (2) Add 5mL of complete culture medium to the cell pellet in step (1), resuspend thoroughly and transfer to a 6cm cell culture dish, place at 37°C, 5% CO 2 cultured in an incubator.
[0086] (3) When the cell density reaches above 80%, 0.05% trypsin can be added to digest the cells. When a small amount of cells fall off the bottom of the dish, immediately add complete culture medium to stop the digestion. Adjust the cell state to be optimal for lentiviral packaging.
[0087] 1.2 Plasmid extracti...
Embodiment 3
[0121] Using the above experimental method, two kinds of CAR-T cells were successfully prepared. The inventors conducted an in vitro killing experiment on HER2-positive tumor cells, and the specific experimental results are as follows.
[0122] 1. Experimental method
[0123] 1.1 Identification of in vitro killing function of Zeta / D-CAR-T cells against HER2-positive tumor cells
[0124] 1.1.1 Dil staining
[0125] (1) HER2 + PC9 and HER2 - PC9 cells were seeded into 48-well plates, each well was seeded with 1×10 5 cells.
[0126] (2) After the cells were cultured overnight, the cell culture medium was aspirated, and the cells were washed 2 times with PBS.
[0127] (3) Add 250 μL of Dil cell membrane staining solution (concentration: 10 μM), shake gently to make the dye evenly cover all the cells.
[0128] (4) Incubate the cells at 37°C in the dark for 20 minutes.
[0129] (5) Aspirate off the cell membrane staining solution and wash 3 times with PBS.
[0130] (6) After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com