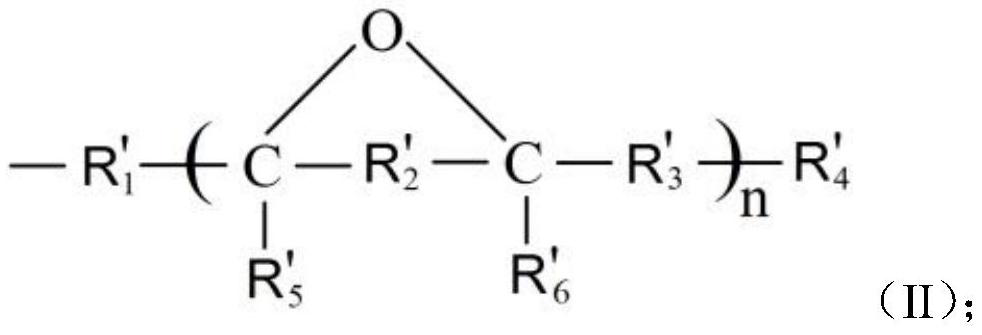
(3, 5-dialkyl-4-hydroxyphenyl) carboxylic acid epoxy alkyl ester compound as well as preparation method and application thereof
A technology of epoxy alkyl ester and hydroxyphenyl, which is applied in lubricating compositions, organic chemistry, additives, etc., can solve the problems of anti-hydrolysis and other problems, and achieve the goals of reducing varieties and dosage, convenient operation, and high reaction conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
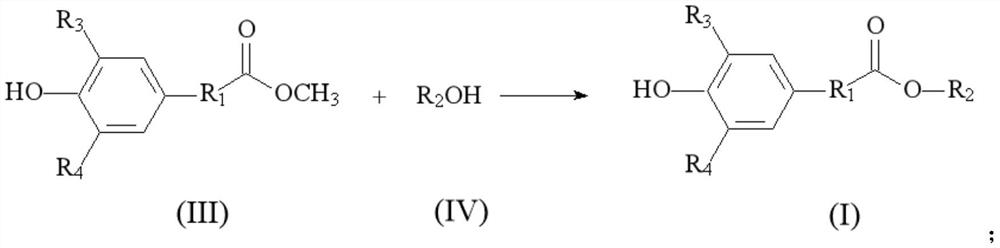
[0072] According to the second embodiment of the present invention, there is provided a method for preparing a (3,5-dihydrocarbyl-4-hydroxyphenyl) carboxylate epoxy alkyl ester compound having the structure of formula (I), the method comprising: The following steps:
[0073] 1) The (3,5-dihydrocarbyl-4-hydroxyphenyl) carboxylate methyl ester compounds with the general structural formula (III) and the epoxidized alkyl alcohol compounds with the general structural formula (IV) are added to the catalyst. The reaction is carried out under the action of , and after purification, a (3,5-dihydrocarbyl-4-hydroxyphenyl) carboxylic acid epoxy alkyl ester compound having the general structural formula (I) is obtained. The specific reaction formula is:
[0074]
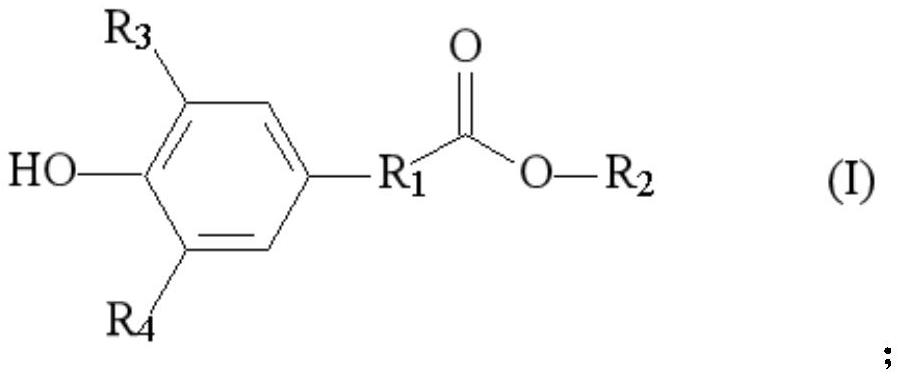
[0075] where R 1 is an alkylene group. R 3 , R 4 Each independently is hydrogen or hydrocarbyl. R 2 It is a hydrocarbon group containing an epoxy bond.
[0076] Preferably, R 1 It is one of alkyl group, alkene group, ...
preparation Embodiment 1
[0090] Put 0.2 mol of glycidol and 0.2 mol of methyl (3,5-di-tert-butyl-4-hydroxyphenyl) propionate into a 250 ml three-necked reaction flask, add 0.4 g of LiOH catalyst, stir and heat. The pressure was reduced to 0.02Mpa, the temperature was 120°C, and the reaction was carried out for 3 hours.
[0091] A viscous material was obtained, light brown in color. The temperature was raised to 260°C, and the unreacted raw materials were distilled off under reduced pressure. A brown-red transparent viscous liquid was obtained. Product conversion was 95.6%.
preparation Embodiment 2
[0093] Put 0.2 mol of glycidol and 0.24 mol of methyl (3,5-di-tert-butyl-4-hydroxyphenyl) propionate into a 250 ml three-necked reaction flask, add 0.4 g of LiOH catalyst, stir and heat. The pressure was reduced to 0.02Mpa, the temperature was 180°C, and the reaction was carried out for 3 hours. A viscous material was obtained, light brown in color. The temperature was raised to 260°C, and the unreacted raw materials were distilled off under reduced pressure. A brown-red transparent viscous liquid was obtained. Product conversion was 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com