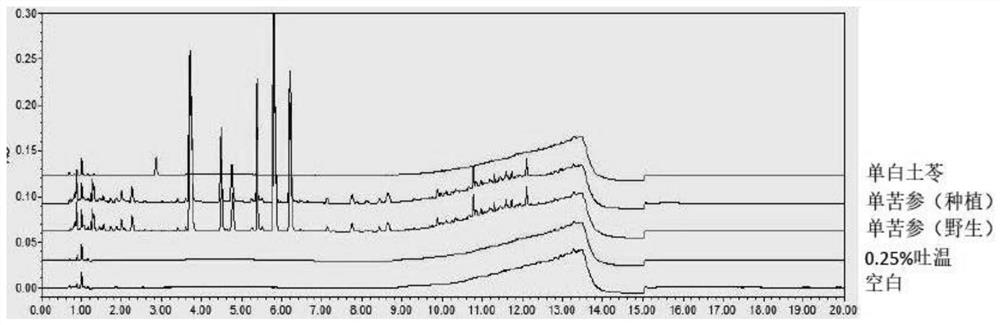
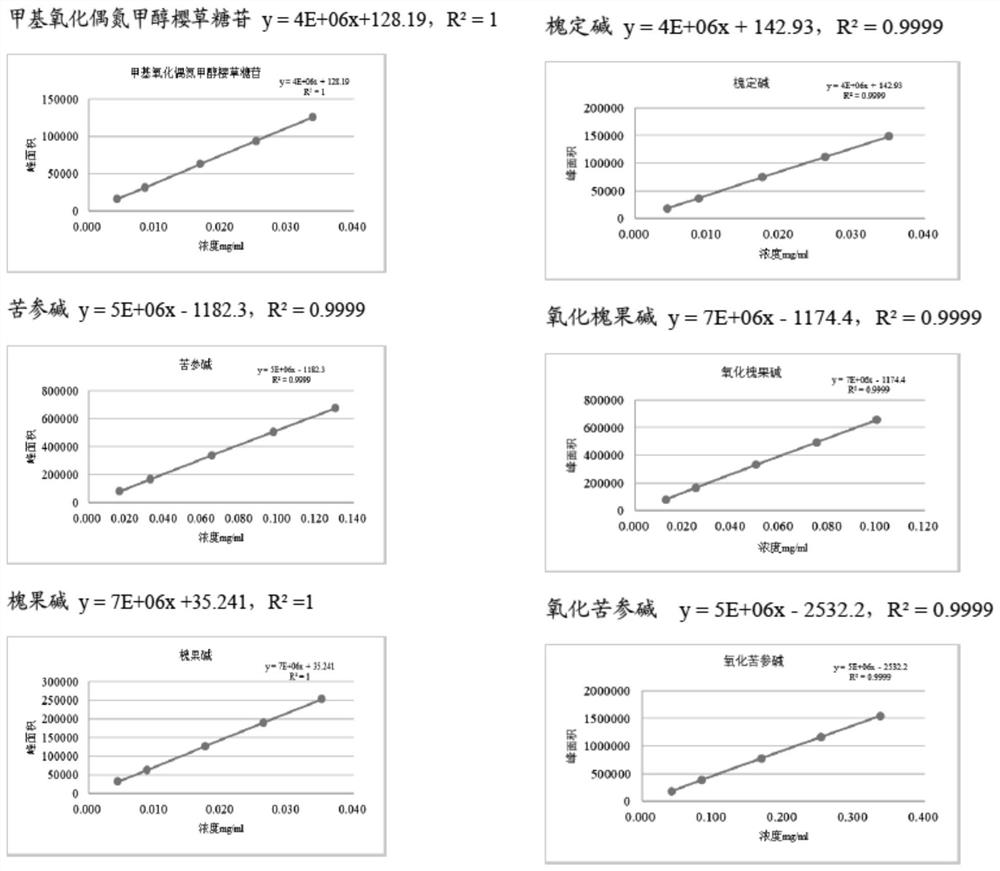
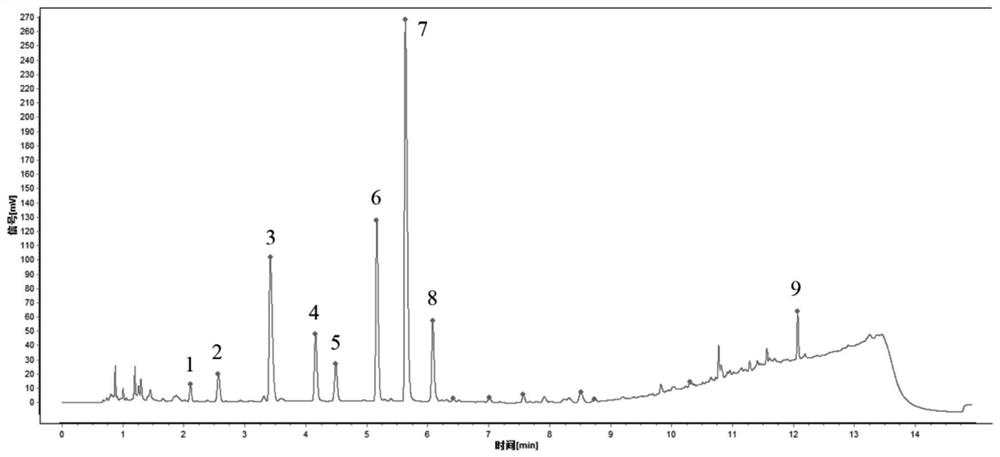
Ultra-high performance liquid chromatography detection method for active ingredient content and fingerprint spectrum of compound sophora flavescens injection
A technology of ultra-high performance liquid phase and Sophora flavescens injection, which is applied in the field of medicine, can solve the problems of poor chromatographic peak shape and inapplicability of compound Sophora flavescens injection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The test article of embodiment 1
[0099] name batch number source Compound Sophora flavescens injection 20181138 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181034 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181139 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181203 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181204 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181209 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181212 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181213 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection 20181214 Shanxi Zhendong Pharmaceutical Co., Ltd. Compound Sophora flavescens injection...
Embodiment 2
[0100] The test article of embodiment 2
[0101]
[0102]
[0103] reagent
[0104] name batch number source level Potassium dihydrogen phosphate 018823 MREAD HPLC Phosphoric acid 0160318 beijing chemical plant Analytical grade methanol 10985407 902 merck gmbh & co. kg HPLC
[0105] Embodiment 1 The detection method of active ingredient of compound Sophora flavescens injection
[0106] 1. Chromatographic conditions, sample preparation, system suitability requirements, calculation formula, limit requirements
[0107]
[0108] 2. Verify the specific content
[0109] 2.1 System suitability
[0110] (1) Experimental steps
[0111] Preparation of blank solution: 0.2% potassium dihydrogen phosphate solution-methanol=85:15 mixed solution, filtered to obtain.
[0112] Preparation of reference substance solution: Precisely weigh matrine reference substance, oxymatrine reference substance and oxymatrine reference substa...
Embodiment 3-1~3-4
[0403] Embodiment 3-1~3-4 UPLC condition exploration
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com