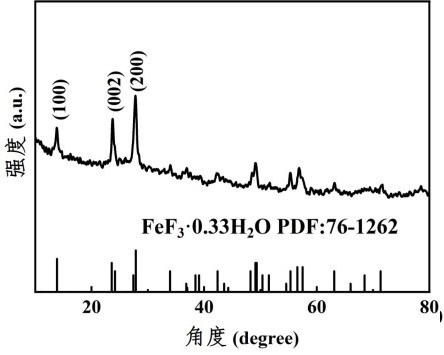
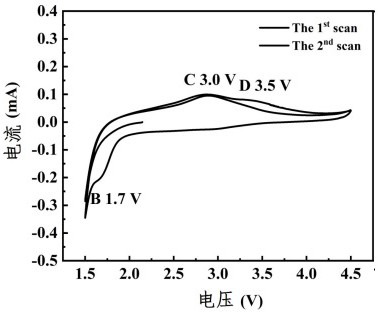
(FeCuZn) F3/rGO composite porous nanomaterial and lithium-fluorine battery
A nanomaterial, lithium-fluorine battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as poor electrical conductivity and affect applications, and achieve good rate performance, effective charge transfer, and superior cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Weigh 20 mg of graphene, add it to 40 ml of ethanol solution, and ultrasonically react for 2 h to make it completely dispersed to obtain a pretreated graphene ethanol dispersion.
[0044] (2) 1350mg Fe(NO 3 ) 3 ·9H 2 O, 45 mg of Cu(CO 3 ) 2 ·3H 2 O, 56 mg of Zn(NO 3 ) 2 ·6H 2 O is dissolved in the graphene ethanol dispersion liquid obtained in step 1), after stirring for 10min, gradually drip the BmimBF of 15ml 4 The ionic liquid (excess of fluoride ions) was stirred at room temperature for 30 min to make it evenly mixed.
[0045] (3) The mixed solution obtained in step 2) was transferred to the reaction kettle, and the mixed solution was cooled to room temperature at a reaction temperature of 120° C. and a reaction time of 14 hours in a vacuum oven using a solvothermal method.
[0046] (4) The precipitated product obtained in 3) was centrifuged, the solid was collected, washed with ethanol for 3 to 5 times, transferred to a vacuum oven, and dried at 80° C....
Embodiment 2
[0049] (1) Weigh 20 mg of graphene, add it to 40 ml of ethanol solution, and ultrasonically react for 2 h to make it completely dispersed to obtain a pretreated graphene ethanol dispersion.
[0050] (2) 1350mg Fe(NO 3 ) 3 ·9H 2 O, 27 mg of Cu(CO 3 ) 2 ·3H 2 O, 78 mg of Zn(NO 3 ) 2 ·6H 2 O, be dissolved in the graphene ethanol dispersion liquid obtained in step 1), after stirring 10min, gradually drip the BmimBF of 15ml 4 The ionic liquid (excess of fluoride ions) was stirred at room temperature for 30 min to make it evenly mixed.
[0051] (3) The mixed solution obtained in step 2) was transferred to the reaction kettle, and the mixed solution was cooled to room temperature at a reaction temperature of 110° C. and a reaction time of 16 h in a vacuum oven using a solvothermal method.
[0052] (4) The precipitated product obtained in 3) was centrifuged, the solid was collected, washed with ethanol for 3 to 5 times, transferred to a vacuum oven, and dried at 80° C. for 12...
Embodiment 3
[0055] (1) Weigh 20 mg of graphene, add it to 40 ml of ethanol solution, and ultrasonically react for 2 h to make it completely dispersed to obtain a pretreated graphene ethanol dispersion.
[0056] (2) 1350mg Fe(NO 3 ) 3 ·9H 2 O, 63 mg of Cu(CO 3 ) 2 ·3H 2 O, 34 mg of Zn(NO 3 ) 2 ·6H 2 O is dissolved in the graphene ethanol dispersion liquid obtained in step 1), after stirring for 10min, gradually drip the BmimBF of 15ml 4 The ionic liquid (excess of fluoride ions) was stirred at room temperature for 30 min to make it evenly mixed.
[0057] (3) The mixed solution obtained in step 2) was transferred to the reaction kettle, and the mixed solution was cooled to room temperature at a reaction temperature of 150 °C and a reaction time of 12 h in a vacuum oven using a solvothermal method.
[0058] (4) The precipitated product obtained in 3) was centrifuged, the solid was collected, washed with ethanol for 3 to 5 times, transferred to a vacuum oven, and dried at 80° C. for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com