Membrane protein dimerization state in situ detection kit and its application
A detection kit and in-situ detection technology, applied in the field of functional nano-probe technology and biological detection, can solve the problem of in situ observation of membrane protein dimerization, cell pathway activation and other problems, achieve high specificity, improve Sensitivity, overcoming the effect of easy photobleaching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of dual recognition probe dual-RP, first SERS probe and second SERS probe
[0055] The H1-Met sequence of the nucleotide chain involved in Example 1 is shown in SEQ ID NO: 5, the H2-Met sequence is shown in SEQ ID NO: 6, the H1-TβRII sequence is shown in SEQ ID NO: 7, and the H2- The sequence of TβRII is shown in SEQ ID NO: 8;
[0056] 1. Preparation of dual-recognition probe dual-RP: 1) After incubating 50 μL solution containing 10 μM H1-Met and H1-TβRII with 500 μL 50 nm AuNPs (0.075 nM) for 12 h at room temperature, aging treatment was performed , that is, adding NaCl solution four times with 30 min intervals each time, the final NaCl concentration is 0.2 M, and the mixture is gently shaken at room temperature for 12 h; 2) Centrifugal washing with PBS (4000 rpm, 20 min) three times. The pellet was resuspended in 50 μL of PBS to obtain dual-RP and stored at 4 °C for further use.
[0057] 2. Preparation of the first SERS probe Met-SERS tag: 1) H...
Embodiment 2
[0060] Example 2 Characterization of the Mechanism of Proximity Hybridization Reaction When Protein Dimerization Occurs
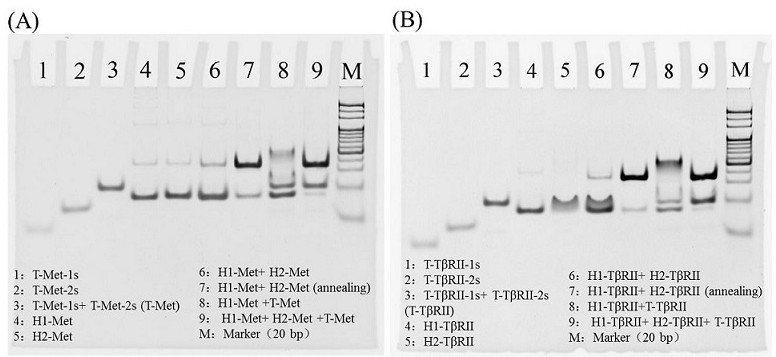
[0061] In order to verify the correctness of the mechanism, a 10% PAGE gel was used for gel electrophoresis experiments. 5 μL of DNA samples were mixed with 1 μL of 6×DNA loading buffer, and the samples were imaged after running at 80 V for 80 min.
[0062] like Figure 4 It is a gel electrophoresis characterization diagram of the present invention to simulate the working mechanism of protein dimerization. In order to simulate the T-Met-1-2 double-stranded T-shaped structure (or T-TβRII-1-2) formed by adjacent hybridization when protein dimerization occurs Double-stranded T-shaped structure), increase the number of complementary bases in the hybridization sequence of T-Met-1 and 2 (or T-TβRII-1 and 2) to obtain T-Met-1s and 2s (or T-TβRII-1s and 2s ), the specific simulation sequence is as follows:
[0063] T-Met-Is as SEQ ID NO: 17 is 5'-AAAAAAAAAAAAAGTG...
Embodiment 3
[0068] Example 3 Assembly of AuNP probes in buffer to form network nanostructures
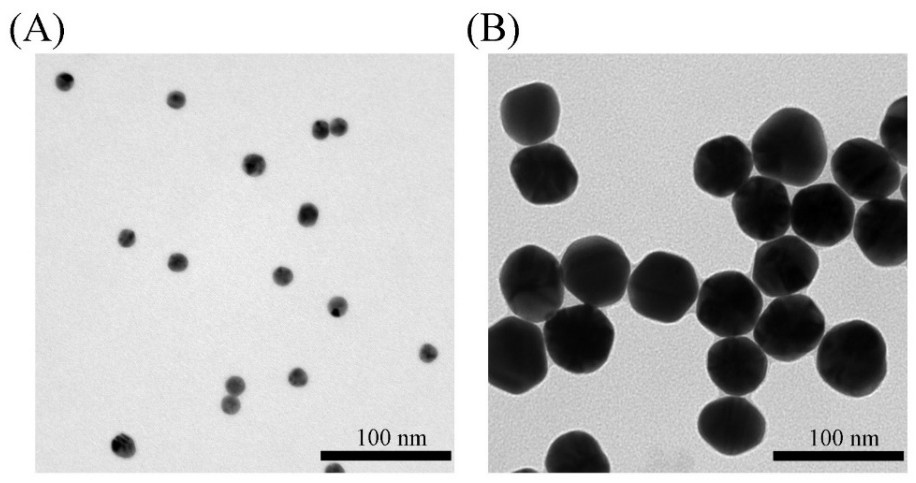
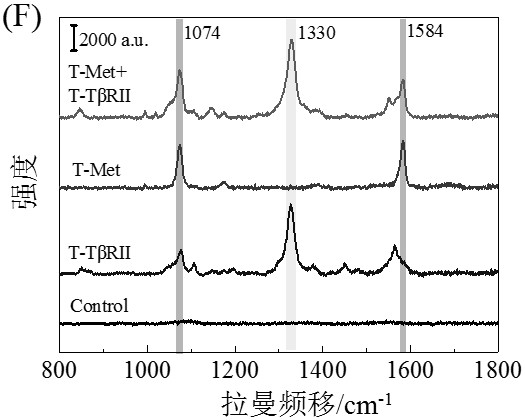
[0069] After mixing an equal amount of 30 μL of Met-SERS tag and TβRII-SERS tag in Example 1, add 60 μL containing 50 nM T mimetic chains, namely T-Met-1s, T-Met-2s, T-TβRII-1s and T -Dual RP of TβRII-2s, followed by incubation at 37 °C with gentle shaking for 2 h to form a 15Au-50Au network nanostructure assembled by 15 nm AuNPs and 50 nm AuNPs. The 15Au-50Au network nanostructures without washing by centrifugation were directly characterized by absorption spectroscopy, TEM and SEM imaging. The SERS spectra of the 15Au-50Au network nanostructures were acquired by a Renishaw system. DLS measurements of nanoparticles were performed after centrifugal washing (3000 rpm, 20 min).
[0070] By characterizing the morphological and optical properties of AuNPs, the assembly of AuNP network nanostructures due to protein dimerization and subsequent SERS sensing were verified. After surface modification...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com