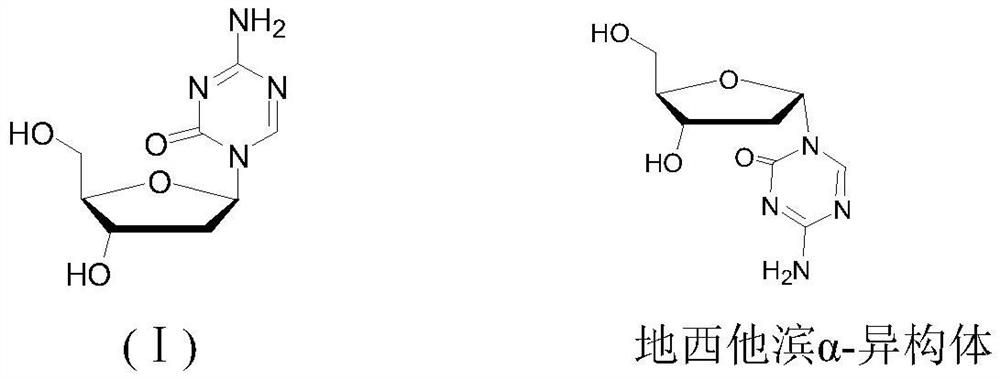
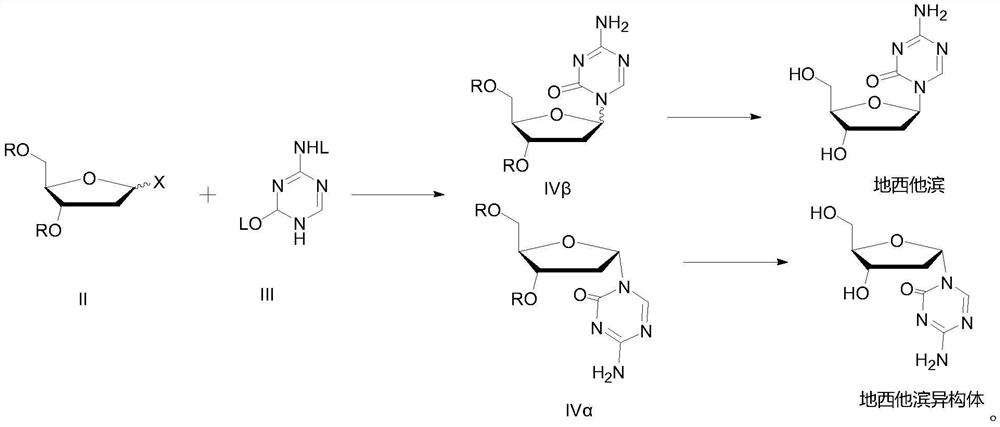
Separation method of decitabine and alpha-type isomer
A decitabine and separation method technology, which is applied in the field of separation and recovery of decitabine and α-isomer, can solve the problem of low recovery efficiency of decitabine, low total yield of decitabine, etc. problem, to achieve the effect of simple and reliable process method, realizing recycling and reducing yield loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take 100g of mother liquor containing decitabine and alpha isomer (content of alpha isomer is 80%), add it to the reaction kettle, add 16L of anhydrous methanol, heat to reflux, continue to add 64L of methyl tert-butyl ether , continue to stir and reflux for 1h, stop heating, add a small amount of seed crystals, naturally stand to cool down to 25°C, crystallize for 4h, filter, and dry to obtain α isomer 65.2g, HPLC: 99.1%.
[0030] The filtrate was concentrated under reduced pressure to 44L, then cooled to 5°C, left to stand for crystallization for 5h, filtered and dried to obtain 16.6g of decitabine, HPLC: 99.3%.
Embodiment 2
[0032] Take 100g of mother liquor containing decitabine and α isomer (α-isomer content 80%), add it to the reaction kettle, add 22 L of anhydrous methanol, heat to reflux, and continue to add 66 L of methyl tert-butyl ether , continue to stir and reflux for 1h, stop heating, add a small amount of seed crystals, naturally stand to cool down to 20°C, crystallize for 3h, filter, and dry to obtain α isomer 64.5g, HPLC: 98.9%.
[0033] The filtrate was concentrated under reduced pressure to 44 L, then cooled to 0° C., left for crystallization for 3 h, filtered, and dried to obtain 17.5 g of decitabine, HPLC: 99.0%.
Embodiment 3
[0035] Take 100g of mother liquor containing decitabine and α isomer (α-isomer content 80%), add it to the reaction kettle, add 20 L of anhydrous methanol, heat to reflux, and continue to add 60 L of methyl tert-butyl ether , continue to stir and reflux for 1h, stop heating, add a small amount of seed crystals, naturally stand to cool down to 25°C, crystallize for 6h, filter, and dry to obtain α isomer 62.6g, HPLC: 99.2%.
[0036] The filtrate was concentrated to 40 L under reduced pressure, then cooled to 5° C., left to stand for crystallization for 6 h, filtered, and dried to obtain 16.7 g of decitabine, HPLC: 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com