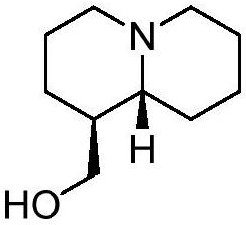
Application of epilupinine and derivative thereof in preparation of medicine for treating depression
A technology of lupine and derivatives, which is applied in the field of medicine, can solve the problems of high social burden risk and suicide risk, and the prevention or treatment of depression that has not been seen before, so as to reduce immobility time and ensure reliability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 10 kg of Lupin (Lupinus micranthus) seeds were crushed and extracted with 95% ethanol under reflux for 3 times, 50 liters each time, and the extraction time was 2 hours each time. The extract was concentrated under reduced pressure to obtain 2.5 kg of extract. The ethanol extract was suspended in 6 liters of distilled water, adjusted to pH 1-3 of the aqueous solution with a 2mol / L dilute hydrochloric acid solution, and extracted 3 times with chloroform, 6 liters each time; the acid aqueous solution was adjusted to pH with a 2mol / L NaOH solution l0-11, extract 3 times with chloroform, each 6 liters. Chloroform was recovered to obtain 80.0 g of total alkaloids. The total alkaloids were crudely fractionated by silica gel column chromatography and eluted with a gradient of chloroform-methanol system (10:0-0:10) to obtain 6 fractions (Frs.A-F). Among them, Fr.B (9.6g) was separated and purified by silica gel column chromatography and Sephadex LH-20 to obtain 2g of compound ...
Embodiment 2
[0054] Take 150 g of epilupinine and appropriate amount of auxiliary materials, sieve, mix well, put into capsules, and make 1000 epilupinine capsules. The content of epilupinine was obtained according to quantitative analysis, which was used as the basis for the dosage.
Embodiment 3
[0056] 150 g of epilupinine was diluted with water and modified with stevioside to prepare a 10% liquid medicine, and the oral liquid of epilupinine was prepared. The content of epilupinine was obtained according to quantitative analysis, which was used as the basis for the dosage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com