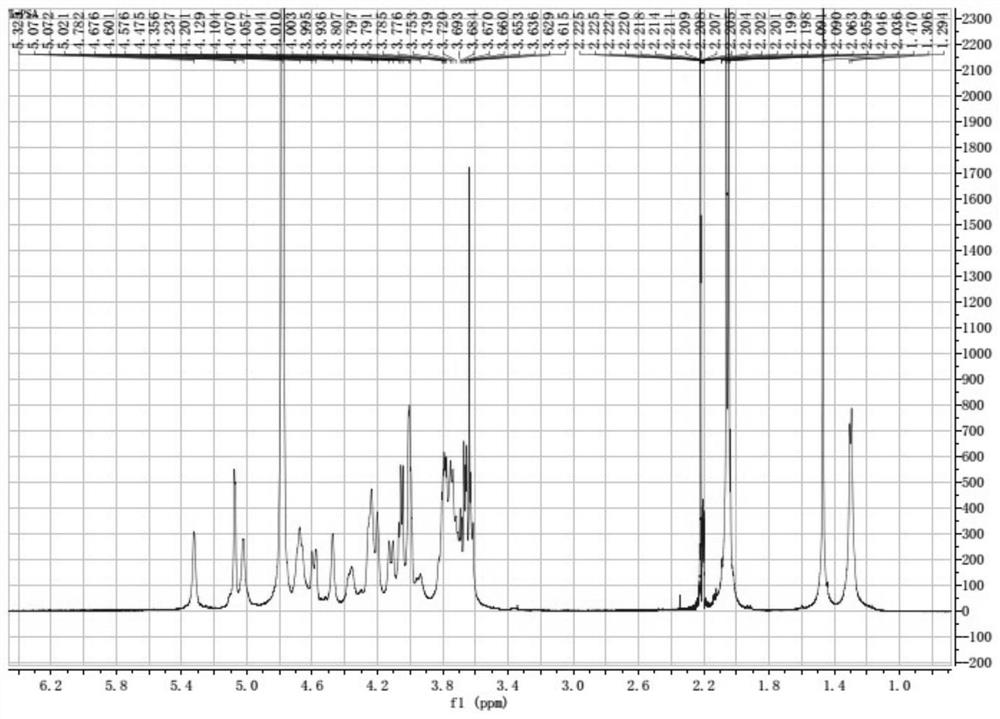
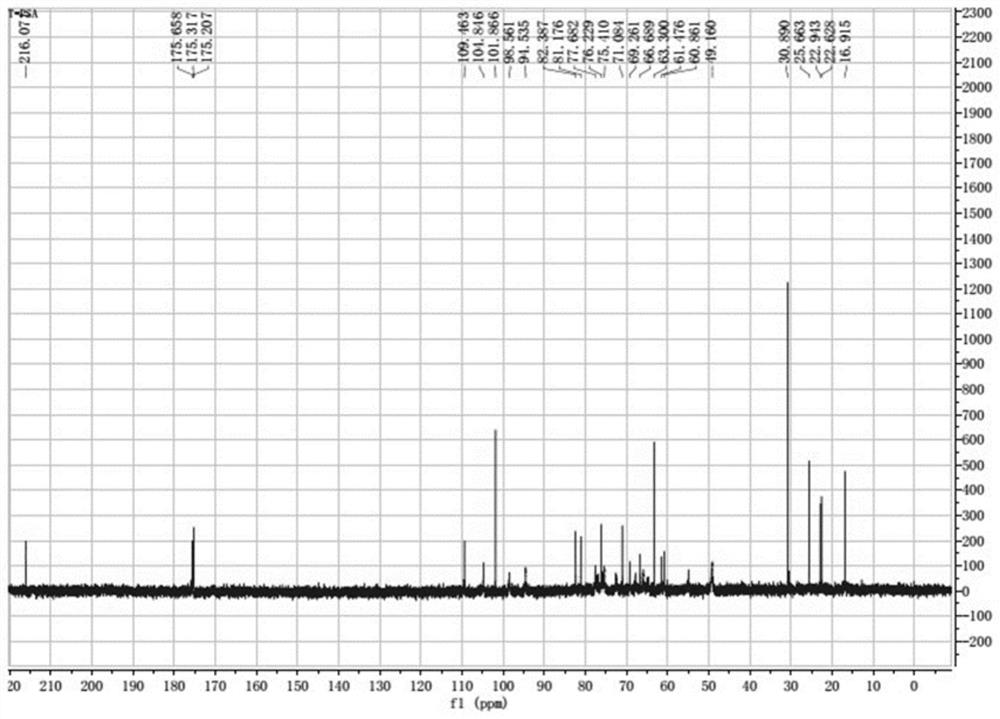
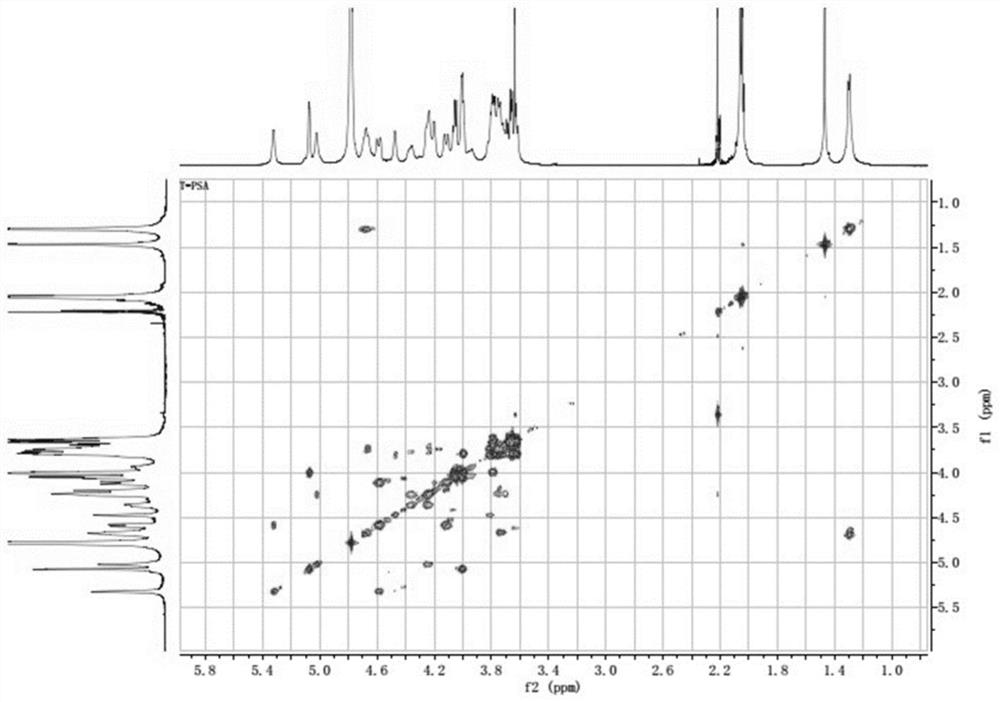
New application of bacteroides fragilis zwitterionic capsular polysaccharide or/and modified zwitterionic capsular polysaccharide
A technology of Bacteroides fragilis and capsular polysaccharide, applied in the field of probiotics, can solve the problems of unsatisfactory treatment effect of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] In some of the embodiments, in step 2), the preparation method of the sulfated reagent includes the following steps: under the conditions of -15 to -10 °C and vigorous stirring, to anhydrous pre-cooled to 0-4 °C Chlorosulfonic acid is added dropwise to the pyridine, and the volume ratio of the chlorosulfonic acid to the anhydrous pyridine is 1:5-10 to obtain a sulfuric acid esterification reagent.
[0081] In some of these embodiments, in step 2), the volume ratio of the suspension to the esterification reagent is 1:2-6;
[0082] In some of the embodiments, in step 2), the conditions for the sulfuric esterification reaction are: 60-90° C. for continuous stirring for 2-4 hours;
[0083] In some of these embodiments, the conditions of the flowing water dialysis in step 2) are: using a 3-10KD dialysis membrane for flowing water dialysis for 24-36 hours; the volume concentration of the ethanol aqueous solution is 80-90%, and the ethanol The time for precipitation of the aq...
Embodiment 1
[0098] Example 1. Preparation of Bacteroides fragilis capsular polysaccharide A
[0099] (1) Streak the strain of Bacteroides fragilis ZY-312 on a blood plate, culture it anaerobically for 48 hours, select a single colony and inoculate it in a plant-derived peptone liquid medium for 8 hours of fermentation (temperature is 37°C), and the obtained bacteria The liquid was centrifuged at 3000 r / min for 15 min, the supernatant was removed, and the precipitate was collected to obtain Bacteroides fragilis ZY-312 bacterial slurry.
[0100] (2) Take 50 g of the bacteria mud prepared in step (1), add 300 g of purified water to resuspend the bacteria, adjust its pH to 3.5 with 1 mol / L hydrochloric acid solution, extract at 100 ° C for 1.5 h, cool to room temperature, and centrifuge at 12000 g at room temperature for 10 min , take the supernatant to get the crude sugar solution.
[0101] (3) The crude sugar solution is concentrated by ultrafiltration through a 10KD ultrafiltration membra...
Embodiment 2
[0105] Example 2. Preparation of sulfated B. fragilis zwitterionic capsular polysaccharide
[0106] (1) The B. fragilis zwitterionic capsular polysaccharide (capsular polysaccharide A) prepared in Example 1 is prepared into a B. fragilis capsular polysaccharide suspension with a concentration of 10 mg / mL with dimethyl sulfoxide;
[0107] (2) adding a sulfating reagent to the suspension obtained in step (1) (the volume ratio of the suspension to the sulfating reagent is 1:2), stirring continuously at 90° C. for 2 h, cooling, adjusting the pH to neutrality, and centrifuging, Collect the supernatant, dialyze the supernatant with a 3KD dialysis membrane under flowing water for 28 hours, then precipitate with an 80% ethanol aqueous solution at 4°C for 12 hours, centrifuge, collect the precipitate, and freeze-dry to obtain a freeze-dried powder;
[0108] (3) The lyophilized powder obtained in step (2) was dissolved in 20 mM Tris-HCl buffer (pH 8.0), and purified by ion exchange chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com