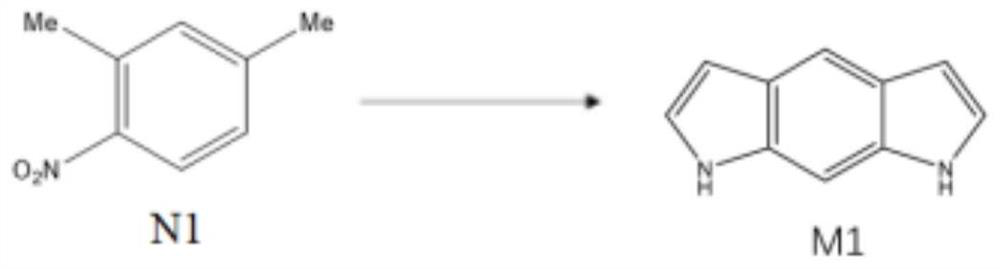
Synthesis method of benzo-p-dipyrrole molecule for hydrogen storage
A technology of a dipyrrole molecule and a synthesis method, which is applied in the field of synthesis of benzo-p-dipyrrole molecules, can solve the problems of low yield, slow efficiency, low output and the like, and achieves the effects of simple and convenient operation, reduced influence and avoided waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
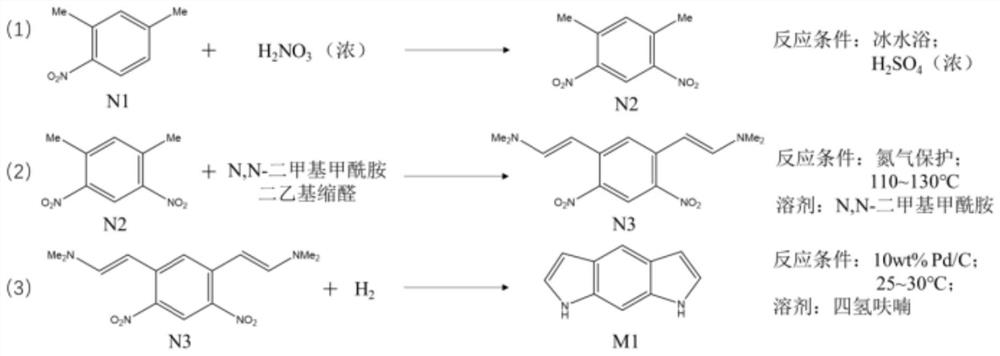
[0076] Step 1: In an ice-water bath, dissolve 10.0 ml of N1 molecules in 20.0 ml of concentrated sulfuric acid, and then dropwise add 6.0 ml of concentrated nitric acid to obtain a solidified first reaction solution. After cooling for 10 minutes, use deionized water for the solidified first reaction solution. After washing, the residual concentrated sulfuric acid and concentrated nitric acid were removed to obtain a solid. The solid was dried in a vacuum drying oven at 60°C for 6 hours, and then recrystallized to obtain about 13.8 g of N2 molecules.
[0077] Step 2: Under nitrogen protection, dissolve 13.8g of N2 molecules in 27.6ml of N,N-dimethylformamide. When the temperature rises to 80°C, add 41.4ml of N,N-dimethylformamide diethyl condensate. Aldehyde, then heated to 110 ° C, reacted for 20 hours and then lowered to room temperature to obtain a second reaction solution, which was naturally filtered to obtain about 17.7 g of N3 molecules.
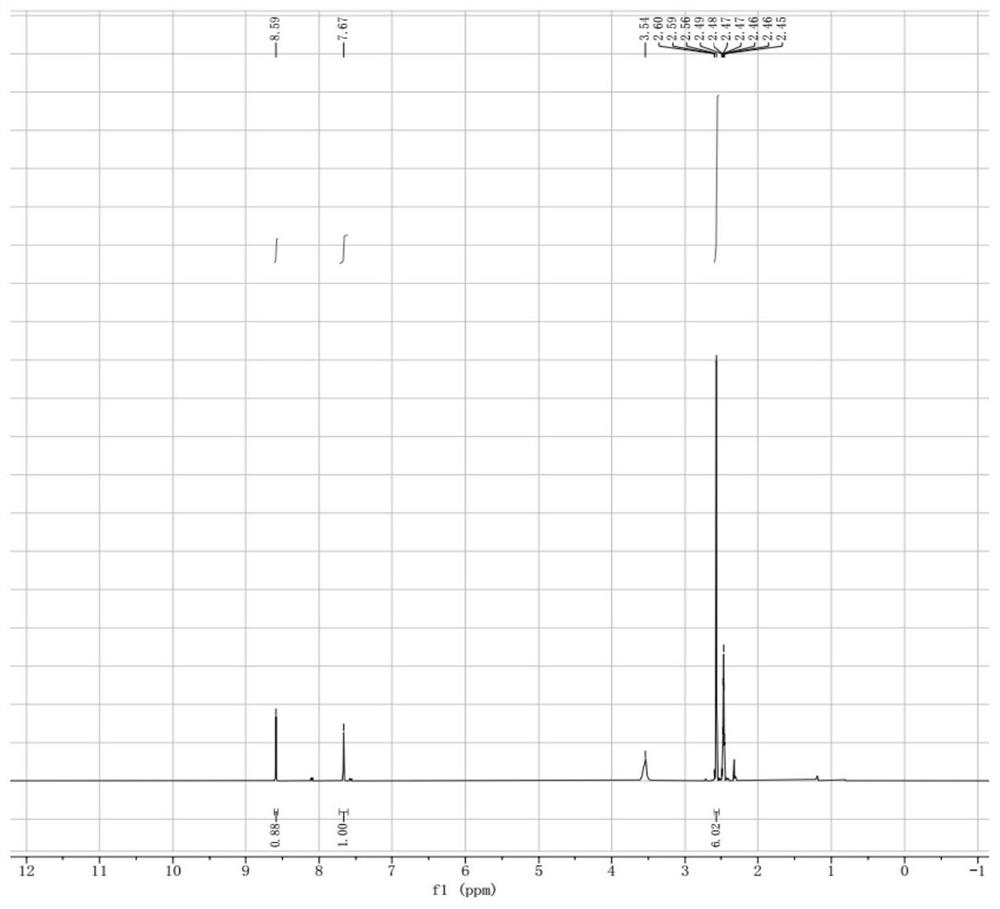
[0078] Step 3: Dissolve 17.7g o...
Embodiment 2
[0080] Step 1: In an ice-water bath, dissolve 5.0 ml of N1 molecules in 12.0 ml of concentrated sulfuric acid, and then dropwise add 5.0 ml of concentrated nitric acid to obtain a solidified first reaction solution, and after cooling for 10 minutes, use deionized water for the solidified first reaction solution After washing, the residual concentrated sulfuric acid and concentrated nitric acid were removed to obtain a solid. The solid was dried in a vacuum drying oven at 60°C for 4 hours, and then recrystallized to obtain about 7.1 g of N2 molecules.
[0081] Step 2: Under nitrogen protection, dissolve 7.1g of N2 molecules in 20.0ml of N,N-dimethylformamide. When the temperature rises to 85°C, add 22.0ml of N,N-dimethylformamide diethyl condensate. Aldehyde, then heated to 115°C, reacted for 24 hours and then lowered to room temperature to obtain a second reaction solution, which was naturally filtered to obtain about 10.0 g of N3 molecules.
[0082] Step 3: Dissolve 10.0g N3 ...
Embodiment 3
[0084] Step 1: Dissolve 1.2ml of N1 molecule in 3.0ml of concentrated sulfuric acid in an ice-water bath, and then add 1.5ml of concentrated nitric acid dropwise to obtain a solidified first reaction solution. After cooling for 5 minutes, use deionized water for the solidified first reaction solution. After washing, the residual concentrated sulfuric acid and concentrated nitric acid were removed to obtain a solid, which was dried in a vacuum drying oven at 60° C. for 3 hours and then recrystallized to obtain about 1.67 g of N2 molecules.
[0085]Step 2: Under nitrogen protection, dissolve 1.67g of N2 molecules in 5.0ml of N,N-dimethylformamide. When the temperature rises to 90°C, add 5.8ml of N,N-dimethylformamide diethyl condensate. Aldehyde, then heated to 120°C, reacted for 20h and then lowered to room temperature to obtain a second reaction solution, which was naturally filtered to obtain about 2.43g of N3 molecules.
[0086] Step 3: Dissolve 2.43g of N3 molecules in 5.0m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com