2, 2-dipyridyl and uracil-1-acetic acid copper tetrafluoroborate complex as well as preparation method and application thereof
A technology of copper tetrafluoroborate tetrafluoroborate and uracil based on acetic acid, applied in the field of copper complexes, can solve the problems of low anticancer activity and the like, and achieve the effects of improving anticancer activity and improving inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] It should be noted that the embodiments of the present invention and the features of the embodiments may be combined with each other under the condition of no conflict. The technical solutions of the present invention will be further described below with reference to the embodiments of the present invention, and the present invention is not limited to the following specific embodiments.
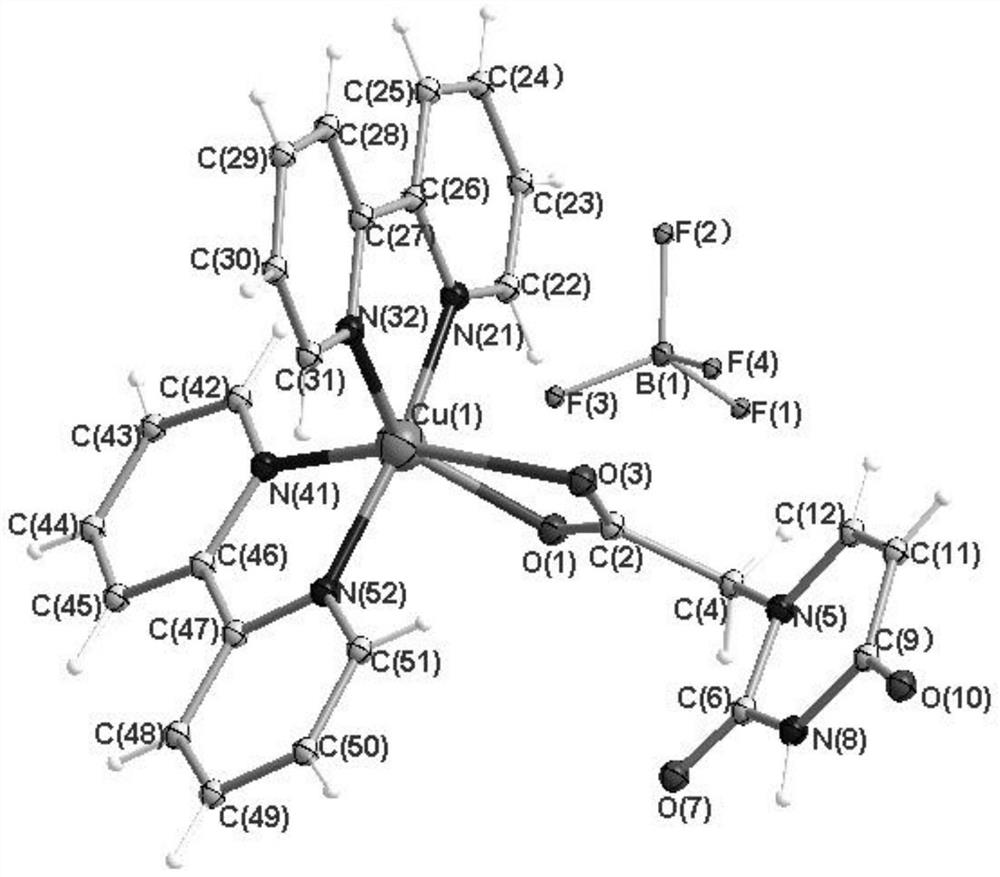
[0042] In one embodiment, a complex of 2,2'-bipyridine and uracil-1-yl acetate tetrafluoroborate copper, the 2,2'-bipyridine and uracil-1-yl acetate copper tetrafluoroborate The structural formula of the complex is:
[0043]
[0044] The 2,2'-bipyridine and uracil-1-ylacetic acid tetrafluoroborate copper complex belongs to the triclinic crystal system, and the space group is P-1; the unit cell parameters are: α=73.5070(10)°, β=89.4130(10)°, γ=61.5200(10)°.
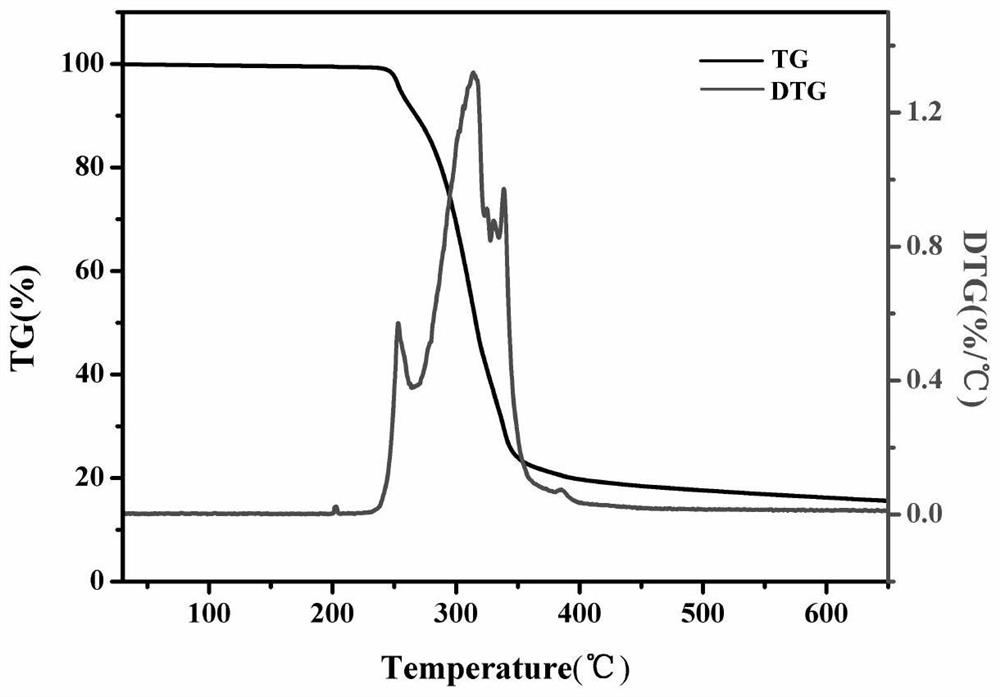
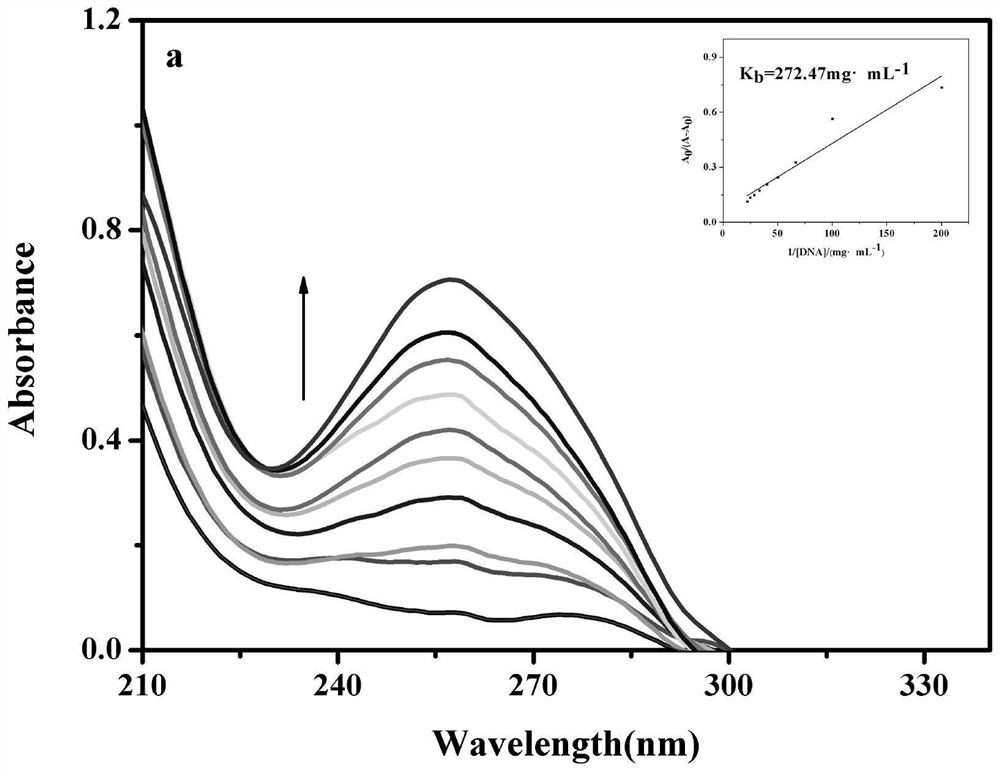
[0045] The performance analysis of the 2,2'-bipyridine and uracil-1-ylacetic acid tetrafluoroborate copper complex (hereina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com