Industrial preparation method of iodixanol
A technology of iodixanol and compounds, which is applied in the field of industrial preparation of iodixanol, can solve the problems of low yield of iodixanol, achieve the effects of improving reaction conversion efficiency, improving reaction efficiency, and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of iodixanol
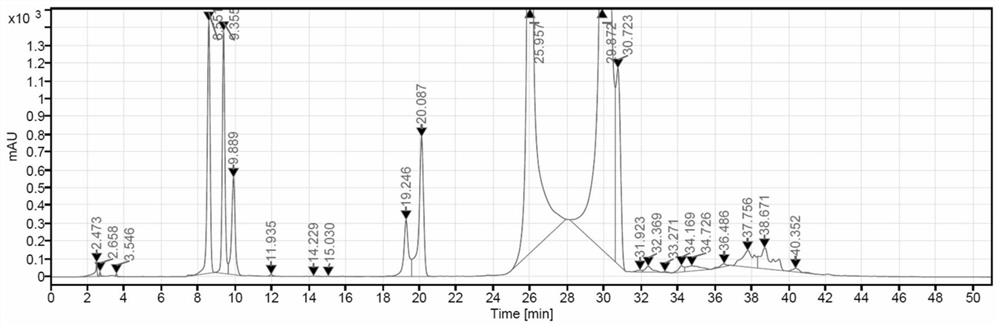
[0036] In a 3000L reactor, epichlorohydrin (49.54kg, 535.43mol) was dissolved in 1000kg dimethylformamide, and the compound of formula II (800kg, 1070.86mol) and zinc chloride (7.3kg, 53.543mol) were added under stirring. Dissolve in, react at room temperature for 5 hours, add 900kg potassium hydroxide solution (25% w / v) to adjust the pH to 6-7, keep stirring for 20 minutes, the HPLC purity of the reaction solution is 71.46%. HPLC spectrum such as figure 1 , the integral data are as follows:
[0037]
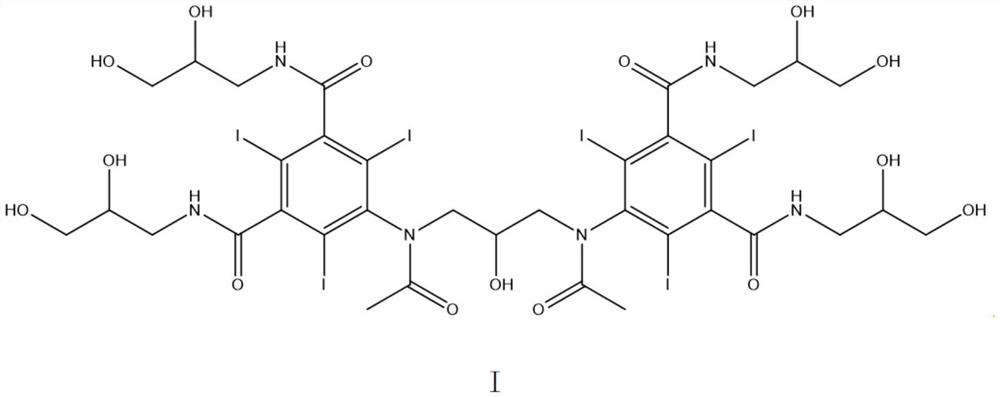
[0038] Those skilled in the art know that the retention time 25.957-29.872 is the peak position of iodixanol. Therefore, to calculate the integral content of iodixanol in the reaction solution, the values in the range of 25.957-29.872 should be added, that is, in figure 1 Among them, the concentration of iodixanol is 27.67%+43.79%=71.46%.
Embodiment 2
[0039] Example 2: Preparation of iodixanol
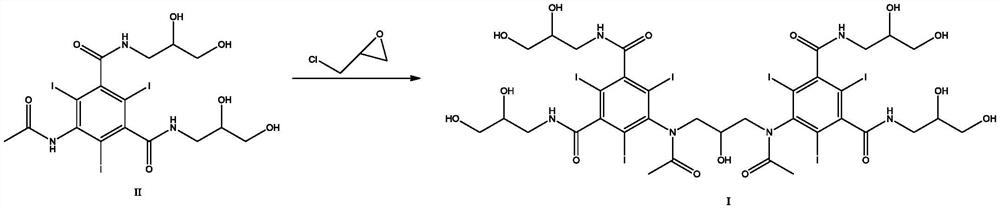
[0040] In a 2000ml reaction flask, epichlorohydrin (33g, 0.36mol) was dissolved in 500g dimethylformamide, and the compound of formula II (533g, 0.71mol) and aluminum chloride (4.74g, 0.0355mol) were added under stirring, The reaction was carried out at room temperature for 3 hours, 600 g of potassium hydroxide solution (25% w / v) was added to adjust the pH to 6 to 7, and the mixture was kept stirring for 10 minutes. The HPLC purity of the reaction solution was 68.59%.
Embodiment 3
[0041] Example 3: Preparation of iodixanol
[0042] In a 2000ml reaction flask, epichlorohydrin (33g, 0.36mol) was dissolved in 500g dimethylformamide, and the compound of formula II (533g, 0.71mol) and ferric chloride (5.76g, 0.0355mol) were added under stirring, The reaction was carried out at room temperature for 3 hours, 600 g of potassium hydroxide solution (25% w / v) was added to adjust the pH to 6-7, and the mixture was stirred for 10 minutes. The HPLC purity of the reaction solution was 69.34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com