Adenovirus vector vaccine for preventing SARS-CoV-2 original strain and Beta strain
A carrier and vaccine technology, applied in the field of virus immunology, can solve the problems of decreased protective effect of Beta mutant strains and inability to achieve the immune effect of Beta strains, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
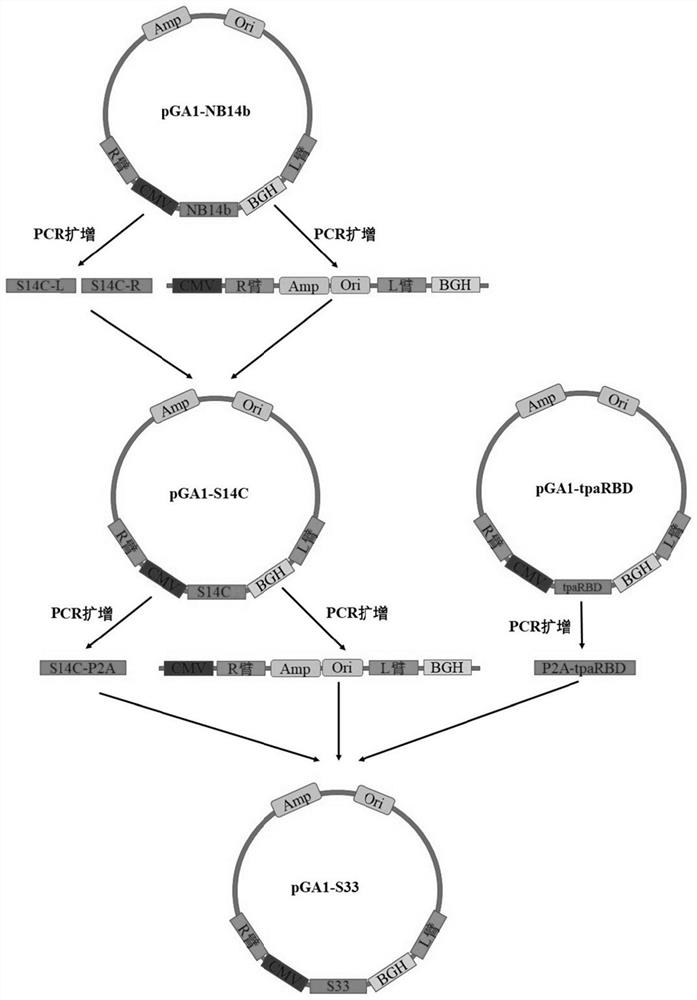
[0060] Example 1 Construction of the double antigen vector pAd5-S33 carrying the S gene of the Beta mutant strain and the RBD gene of the original strain
[0061] 1. Construction of the shuttle plasmid pGA1-S14c of the S gene of the Beta mutant strain
[0062] Using PGA1-NB14b (British mutant strain S gene vector, its sequence is obtained by point mutation on the original strain sequence of SEQ ID NO. Point mutation, which is obtained by adding the amino acids KV at positions 986 to 987 in the S2 region to PP; the above mutations promote the protein to maintain the pre-fusion conformation, thereby inducing neutralizing antibodies; the sequence of the NB14b is shown in SEQ ID NO: 2 ; preserved by Guangzhou Enbao Biomedical Technology Co., Ltd.) plasmid as template. The target fragment S14C-L was obtained by PCR amplification with Primer Star Mix (TaKaRa) using S-F and K417N-R as primers.
[0063] S14C-L amplification primer sequence:
[0064] S-F: ggtaccgagctcggatccgccaccatg...
Embodiment 2
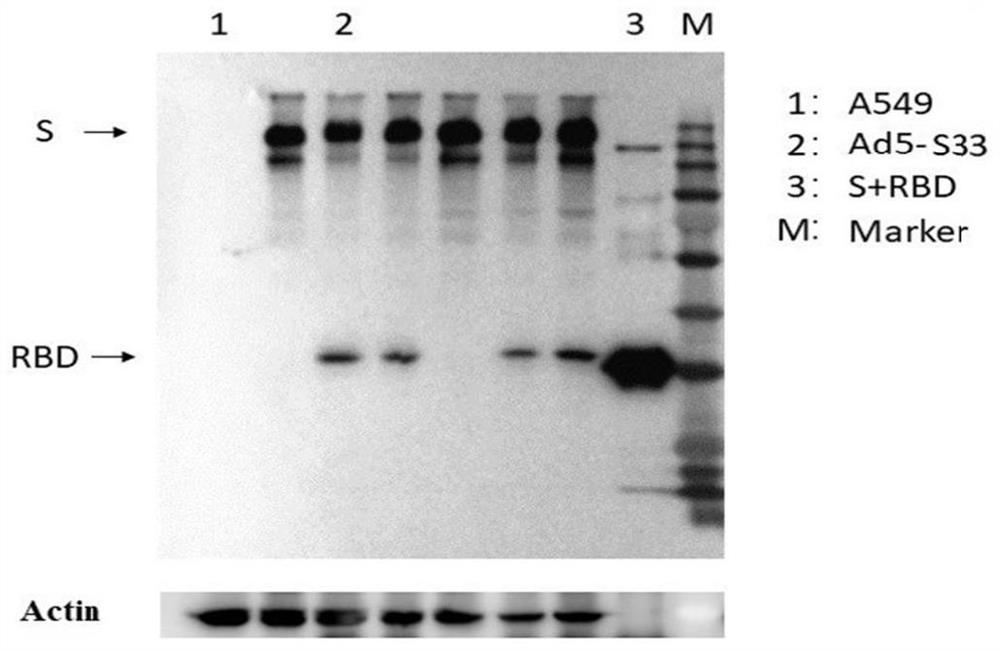
[0102] Example 2 Adenovirus vector vaccine against SARS-CoV-2 original strain and Beta strain
[0103] 1. Rescue and production of Ad5-S33 vector
[0104] According to the conventional method, Ad5-S33 was linearized with PacI, recovered by ethanol precipitation, and transfected into 293 cells by cationic lipofection method. 4 hours after transfection, 2 ml of DMEM medium containing 5% fetal bovine serum was added, and incubated for 7 hours. -10 days, observe the cytopathic changes; after the poisoning, collect the cells and the culture supernatant, freeze and thaw 3 times in a 37-degree water bath and liquid nitrogen, and centrifuge to remove cell debris, and the supernatant infects a 10 cm dish; 2-3 days later, Collect cells and culture supernatant, freeze and thaw 3 times and centrifuge to remove cell debris, and infect 3-5 15 cm dishes with supernatant; after 2-3 days, collect cells, freeze and thaw 3 times and centrifuge to remove cell debris; supernatant After infecting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com