Variant dihydropicolinic acid reductase polypeptides and method for producing L-threonine using same
A technology of dihydrodipicolinate and reductase, which is applied in the field of mutant polypeptides, can solve the problems of growth inhibition of bacterial strains, and achieve the effect of increasing the production of threonine and reducing the production of lysine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Preparation of a vector library for introducing modifications into the dapB gene ORF
[0110] In order to find a variant in which the expression level of the dapB gene of C. glutamicum or its activity is attenuated, a library was prepared by the following method.
[0111] First, to introduce 0-4.5 modifications per kb to the DNA fragment (747 bp) containing the dapB (747 bp) gene, the Genemorph II Random Mutagenesis Kit (Stratagene) was used. Error-prone PCR was performed using chromosomal DNA of Corynebacterium glutamicum ATCC13032 (WT) as template and primer pair of SEQ ID NOs: 5 and 6. Specifically, chromosomal DNA (500 ng) comprising WT strain, primer pair of SEQ ID NO: 5 and 6 (125 ng each), Mutazyme II reaction buffer (1Х), dNTP mixture (40 mM) were mixed The reaction mixture with Mutazyme II DNA polymerase (2.5U) was denatured at 94°C for 2 minutes, followed by 25 cycles (denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, Polymerizat...
Embodiment 2
[0113] Example 2: Preparation of dapB deletion strains and screening of dapB mutant strains based on growth rate
[0114] In order to prepare a dapB gene-deleted strain from wild-type Corynebacterium glutamicum ATCC13032, pDZ-ΔdapB as a dapB gene deletion vector was prepared as follows. Specifically, DNA fragments (300 bp each) located at the 5'- and 3'-ends of the dapB gene were ligated into the pDZ vector (Korean Patent No. 2009-0094433). Based on the reported nucleotide sequence of the dapB gene (SEQ ID NO: 2), primers of SEQ ID NOS: 7 and 8 in which restriction enzyme SalI recognition sites were inserted at the 5'- and 3'-ends were synthesized, and the Primers of SEQ ID NO: 9 and 10 at 300 bp away. The 5'-end gene fragment was prepared by PCR using the chromosomal DNA of Corynebacterium glutamicum ATCC13032 as a template and the primer pair of SEQ ID NOS: 7 and 9. In the same manner, a gene fragment of the 3'-end of the gltA gene was prepared by PCR using the primer pa...
Embodiment 3
[0125] Example 3: Sequencing of 6 dapB mutant strains
[0126] To identify the dapB gene sequences of the selected 6 strains (ie, 13032::dapB(mt)-1 to 6), the primers specified in Example 1 (SEQ ID NOS: 5 and 6) were used to amplify by PCR Each DNA segment in the chromosome that contains the dapB gene. PCR conditions were as follows: denaturation at 94°C for 2 min, followed by 30 cycles (denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and polymerization at 72°C for 40 seconds), followed by polymerization at 72°C for 10 minute.
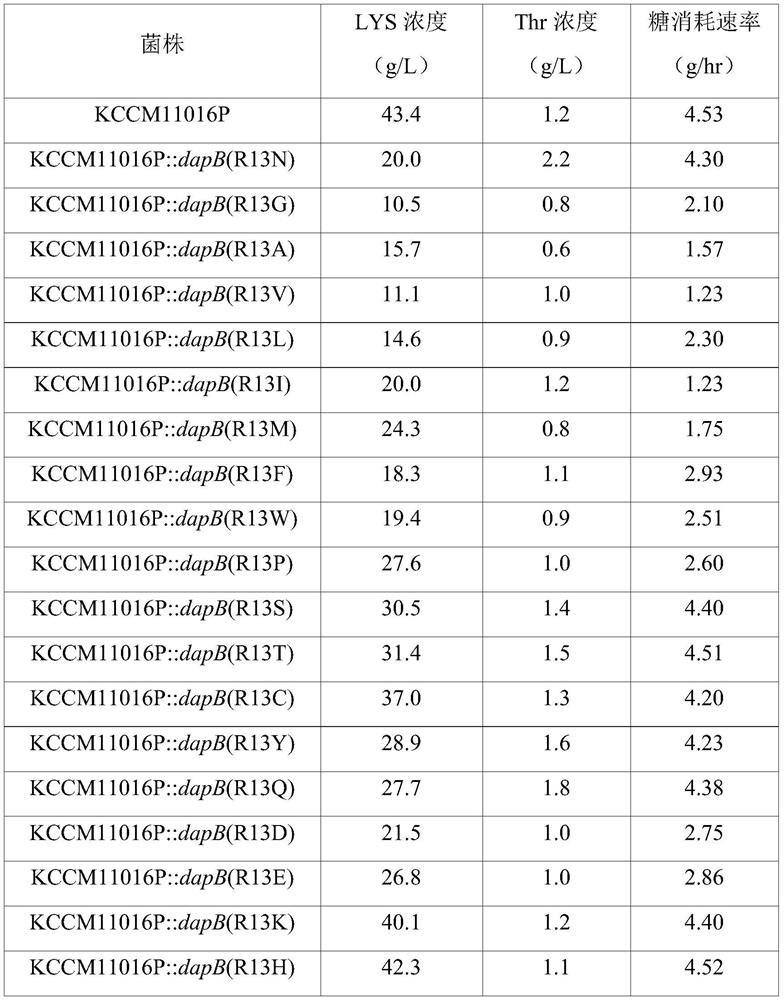
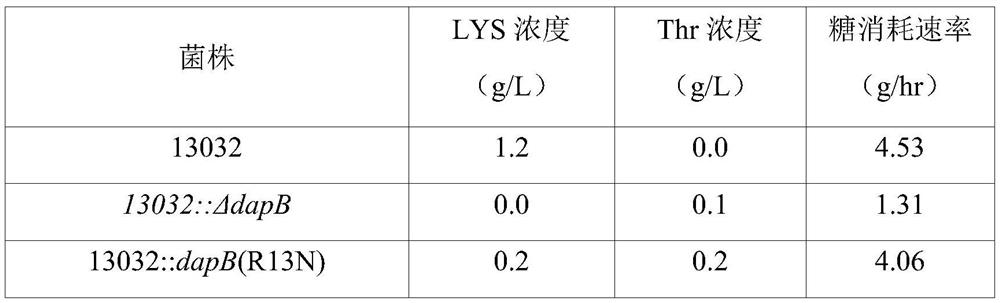
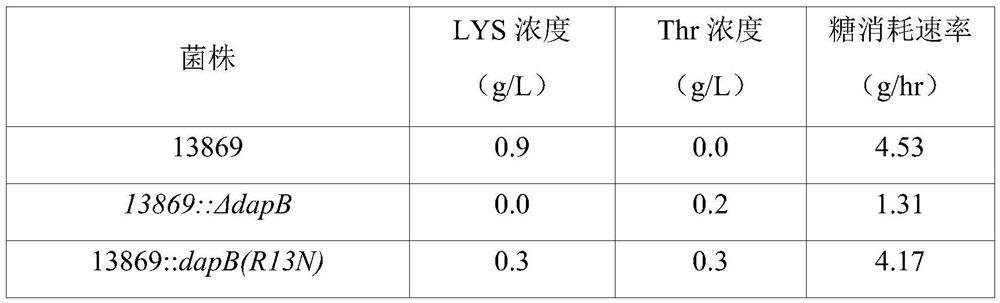
[0127]As a result of analyzing the sequence of the amplified gene, it was confirmed that among the 6 strains, 13032::dapB(mt)-1 was the one in which the existing CGT at positions 37 to 39 of SEQ ID NO: 2 was replaced by AAC and A variant in which the amino acid at position 13 from its N-terminus (ie, arginine) is replaced by asparagine; 13032::dapB(mt)-2 is where the amino acid at positions 106 to 108 of SEQ ID NO:2 is A variant in wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com