Synthesis method of Tirzeptide
A synthesis method and fmoc-asp technology, applied in the synthesis field of Tirzepatide, can solve problems such as unfavorable industrial production, complicated operation steps, high cost, etc., and achieve the advantages of improving crude peptide purity, high promotion value, reducing material cost and purification cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
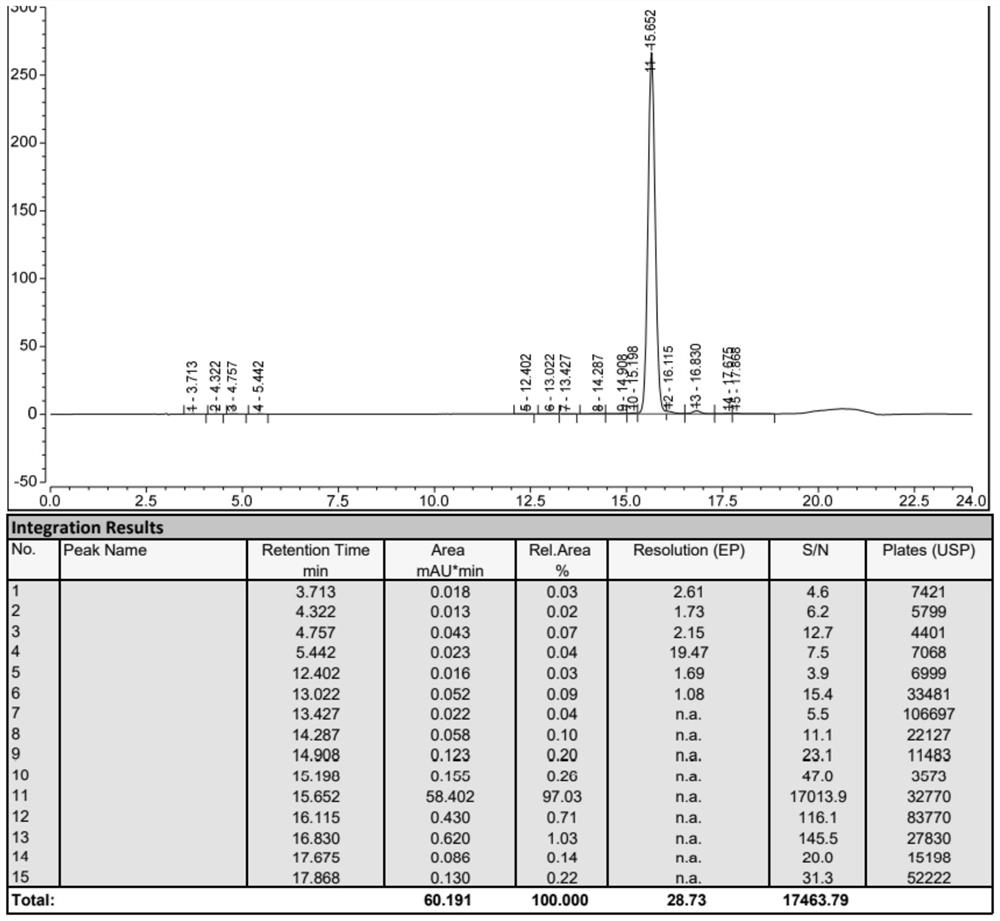
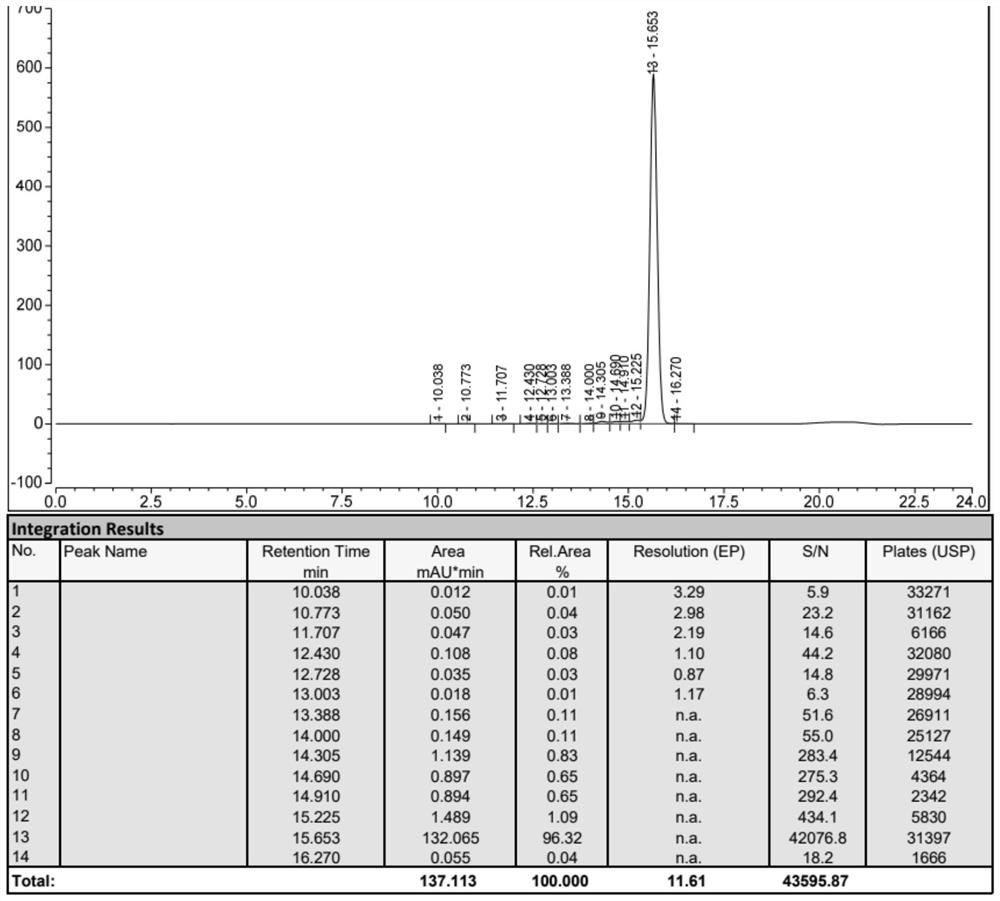
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of Fmoc-Thr(tBu)-Ser(Psi(Me,Me)pro)-OH Tirzepatide peptide resin:
[0046] Step 1, the 7th-8th amino acid of tirzepatide sequence is used as pseudo-pro dipeptide Fmoc-Thr(tBu)-Ser(Psi(Me,Me)pro)-OH;
[0047] In step 2, 30.00 g (Sub=0.33 mmol / g) of Rink Amide-MBHA resin was weighed into the solid-phase reactor, and 200 mL of DCM was added to swell the resin for 0.5 h. The solvent was drained, 200 mL v / v (volume ratio) 20% piperidine / DMF solution was added, that is, a DMF solution containing 20% piperidine by volume, and the deprotection reaction was performed for 10+20 min. Suction dry, add DMF 250mL to wash 6 times. The indene test was positive. Weigh 11.387 g of Fmoc-Ser(tBu)-OH, 4.82 g of HOBT, 5.5 mL of DIC, and 200 mL of DMF solution, activate in ice bath for 10 minutes, and the activation temperature does not exceed 10 degrees Celsius. The activated solution was added to the reactor and reacted for 1 hour. After the indene test result wa...
Embodiment 2
[0059] Example 2: Preparation of Fmoc-Tyr(tBu)-Ser(Psi(Me,Me)pro)-OH Tirzepatide Peptide Resin:
[0060] Step 1: use the 10th-11th amino acids of the tirzepatide sequence as the pseudo-pro dipeptide Fmoc-Tyr(tBu)-Ser(Psi(Me,Me)pro)-OH;
[0061]In step 2, 30.00 g (Sub=0.33 mmol / g) of Rink Amide-MBHA resin was weighed into the solid-phase reactor, and 200 mL of DCM was added to swell the resin for 0.5 h. The solvent was drained, 200 mL v / v (volume ratio) 20% piperidine / DMF solution was added for deprotection, and the reaction was performed for 10+20 min. Suction dry, add DMF 250mL to wash 6 times. The indene test was positive. Weigh 11.387 g of Fmoc-Ser(tBu)-OH, 4.82 g of HOBT, 5.5 mL of DIC, and 200 mL of DMF solution, activate in ice bath for 10 minutes, and the activation temperature does not exceed 10 degrees Celsius. The activated solution was added to the reactor and reacted for 1 hour. After the indene test result was negative, it was drained. Add DMF to wash 3 times,...
Embodiment 3
[0070] Example 3: Preparation of Fmoc-Ser(tBu)-Ser(Psi(Me,Me)pro)-OH Tirzepatide Peptide Resin:
[0071] Step 1, take the 32-33rd amino acid of tirzepatide sequence as pseudo-pro dipeptide Fmoc-Ser(tBu)-Ser(Psi(Me,Me)pro)-OH;
[0072] In step 2, 30.00 g (Sub=0.33 mmol / g) of Rink Amide-MBHA resin was weighed into the solid-phase reactor, and 200 mL of DCM was added to swell the resin for 0.5 h. The solvent was drained, 200 mL v / v (volume ratio) 20% piperidine / DMF solution was added for deprotection, and the reaction was performed for 10+20 min. Suction dry, add DMF 250mL to wash 6 times. The indene test was positive. Weigh 11.387 g of Fmoc-Ser(tBu)-OH, 4.82 g of HOBT, 5.5 mL of DIC, and 200 mL of DMF solution, activate in ice bath for 10 minutes, and the activation temperature does not exceed 10 degrees Celsius. The activated solution was added to the reactor and reacted for 1 hour. After the indene test result was negative, it was drained. Add DMF to wash 3 times, each 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com