Haploid low-alkalinity hindered amine light stabilizer and preparation method thereof
A hindered amine light stabilizer and monomer type technology, applied in the field of hindered light stabilizers, can solve the problems of limiting the synergistic stabilization effect of phenolic antioxidants and high alkalinity, and achieve suitable preparation cost, low alkalinity, and light stability. Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
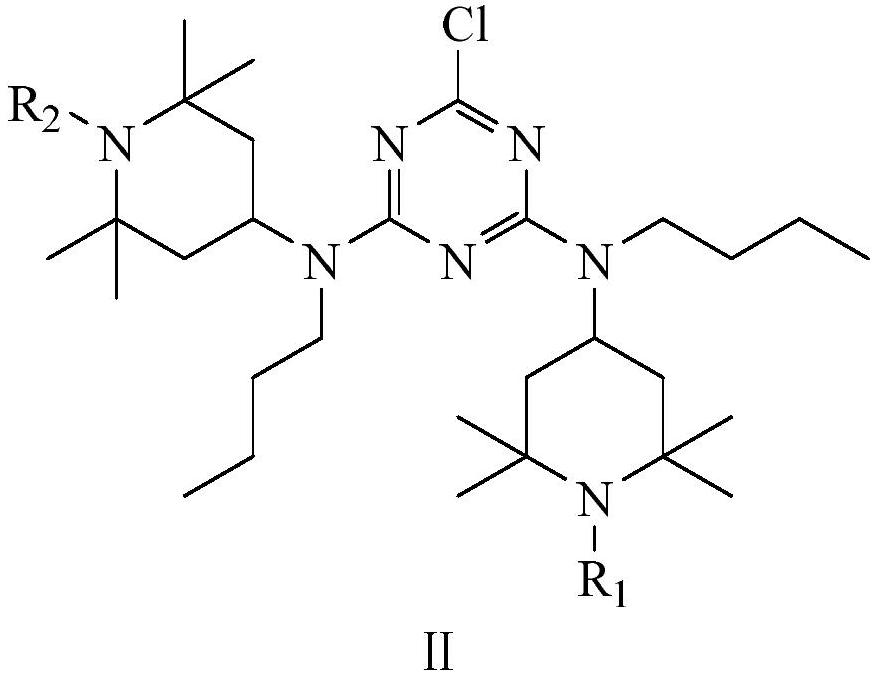
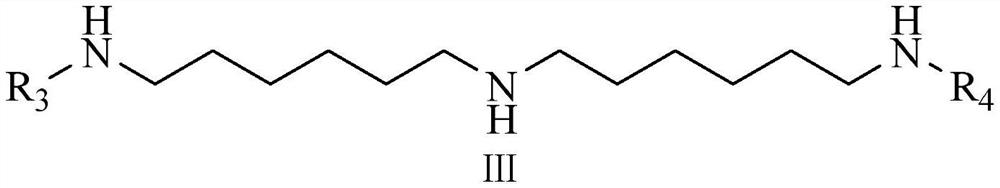
[0035]To a 2L autoclave equipped with mechanical magnetic stirring was added bis(hexamethylene)triamine (37.69 g, 0.175 mol), 4,6-bis(N-butyl-2,2,6,6-tetramethyl-4 -Piperidinylamino)-2-chloro-1,3,5-triazine (257.94g, 0.481mol), sodium hydroxide (20.2g, 0.505mol), water (80mL) and 500mL xylene as solvent, nitrogen Fully replace, turn on stirring, heat up to 170°C, and react with heat preservation and pressure for 18 hours; after heat preservation, cool down to 80°C, separate water, wash with water, filter, and distill under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified to obtain 249.34 g of product, the light transmittance was 96.5%, and the yield was 89.67%.
[0036] 1 H-NMR(DMSO, 300HZ): δ0.95(t, 18H), 1.23(s, 72H), 1.27-1.3(br, m, 8H), 1.3-1.38(m, 12H), 1.4-1.47(m ,12H),1.51-1.55(br,16H),1.6-1.68(br,m,4H),1.7-1.74(dd,m,12H),2.05(s,6H),2.65(m,6H),3.35 (m, 4H), 3.5 (t, 16H), 7.0 (br, 2H).
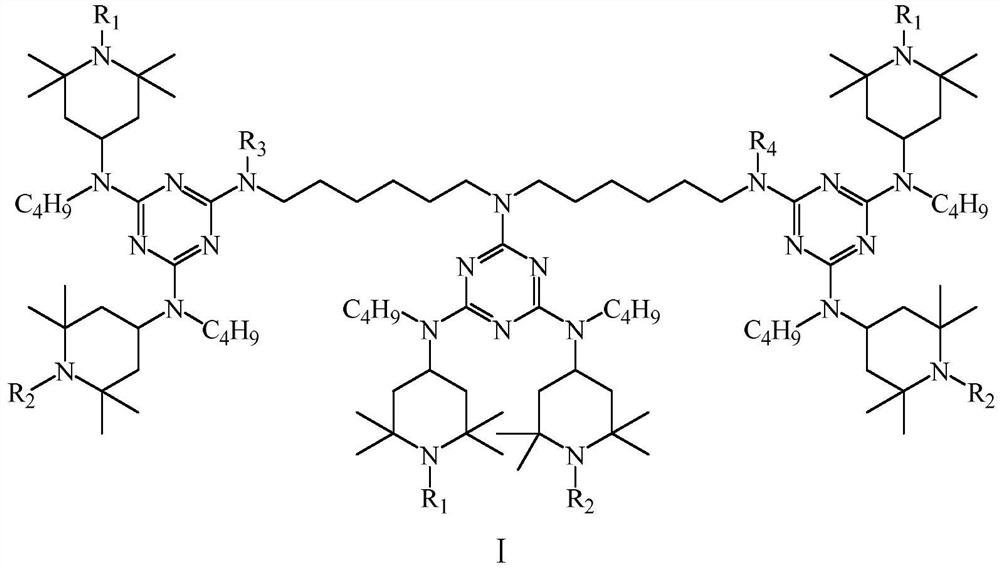
[0037] The structural formula is...
Embodiment 2
[0040] To a 2L autoclave equipped with mechanical magnetic stirring was added bis(hexamethylene)triamine (33.33 g, 0.169 mol), 4,6-bis(N-butyl-2,2,6,6-tetramethyl-4 -Piperidinylamino)-2-chloro-1,3,5-triazine (257.94g, 0.481mol), sodium hydroxide (20.2g, 0.505mol), water (80mL) and 385mL xylene as solvent, nitrogen Fully replace, turn on stirring, heat up to 140°C, and react with heat preservation and pressure for 24 hours; after heat preservation, cool down to 80°C, separate water, wash with water, filter, and distill under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified to obtain 253.34 g of product, the light transmittance was 96.1%, and the yield was 90.07%.
[0041] 1 H-NMR (DMSO, 300HZ): δ0.94 (t, 18H), 1.23 (s, 72H), 1.27-1.31 (br, m, 8H), 1.33-1.38 (m, 12H), 1.41-1.46 (m) ,12H),1.52-1.56(br,16H),1.61-1.68(br,m,4H),1.69-1.74(dd,m,12H),2.05(s,6H),2.65(m,6H),3.34 (m, 4H), 3.5 (t, 16H), 7.02 (br, 2H).
Embodiment 3
[0043] To a 2L autoclave equipped with mechanical magnetic stirring was added bis(hexamethylene)triamine (31.54 g, 0.161 mol), 4,6-bis(N-butyl-2,2,6,6-tetramethyl-4 -Piperidinylamino)-2-chloro-1,3,5-triazine (257.94g, 0.481mol), sodium hydroxide (20.2g, 0.505mol), water (80mL) and 320mL xylene as solvent, nitrogen Fully replace, turn on stirring, heat up to 200°C, and react with heat preservation and pressure for 12 hours; after heat preservation, cool down to 80°C, separate water, wash with water, filter, and distill under reduced pressure to remove the solvent to obtain a crude product. The crude product was purified to obtain 242.34 g of the product, with a light transmittance of 96.8% and a yield of 87.57%.
[0044] 1 H-NMR(DMSO, 300HZ): δ0.95(t, 18H), 1.23(s, 72H), 1.27-1.3(br, m, 8H), 1.3-1.38(m, 12H), 1.4-1.47(m ,12H),1.51-1.55(br,16H),1.6-1.68(br,m,4H),1.7-1.74(dd,m,12H),2.05(s,6H),2.65(m,6H),3.35 (m, 4H), 3.5 (t, 16H), 7.0 (br, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com