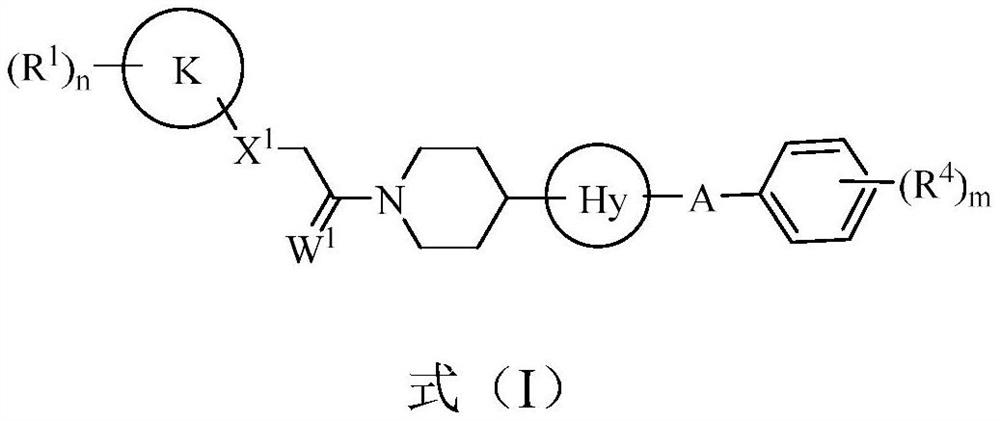
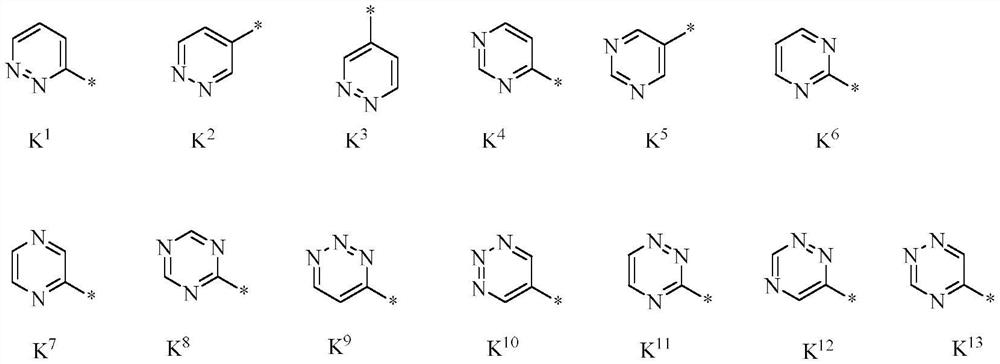
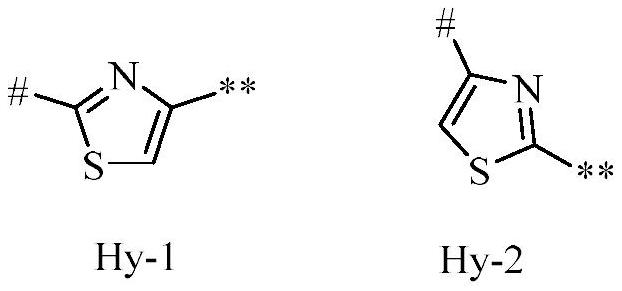
1-(4-(4-(5-phenyl-4, 5-dihydroisoxazol-3-yl) thiazol-2-yl) piperidin-1-yl)-ethane-1-one derivatives and related compounds as fungicides for crop protection
A compound, C1-C6 technology, applied in the control or prevention of phytopathogenic fungi, the preparation of 6-membered heteroaryl piperidinyl ethyl ketone, the field of intermediates of 6-membered heteroaryl piperidinyl ethyl ketone, Can solve the problem of toxicity, residue formation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0311] Example 1: 1-(4-(4-(5-(2,6-dichlorophenyl)-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)piperidine-1- Preparation of yl)-2-((3-(trifluoromethyl)pyrazin-2-yl)oxy)ethane-1-one (compound 3)
[0312] Step A: Preparation of ethyl 2-bromo-1,3-thiazole-4-carboxylate
[0313] To a solution of ethyl 2-aminothiazole-4-carboxylate (100 g, 581 mmol) and copper(II) bromide (195 g, 871 mmol) in acetonitrile (1 L) at 0 °C was added tert-butyl nitrite (104 mL) dropwise , 871 mmol)). The reaction mixture was heated to 25°C and stirred for 12 hours. The reaction mixture was diluted with ethyl acetate (1 L) and water (3 L) and acidified to pH 2 using 1 N hydrochloric acid. The layers were separated and the aqueous layer was extracted three times with ethyl acetate (500 mL). The combined ethyl acetate layers were dried over anhydrous sodium sulfate, concentrated, and purified by recrystallization from hexane to obtain ethyl 2-bromo-1,3-thiazole-4-carboxylate (115 g, 84% yield). ).
[0314] ...
example 2
[0340] Example 2: 3-Chloro-2-(3-(2-(1-(2-((3-(trifluoromethyl)pyrazin-2-yl)oxy)acetyl)piperidin-4-yl) Preparation of Thiazol-4-yl)-4,5-dihydroisoxazol-5-yl) phenylmethanesulfonate
[0341] Step A: 3-Chloro-2-(3-(2-(1-(2-((3-(trifluoromethyl)pyrazin-2-yl)oxy)acetyl)piperidin-4-yl ) thiazol-4-yl)-4,5-dihydroisoxazol-5-yl) phenylmethanesulfonate preparation
[0342] To (E / Z)-tert-butyl 4-(4-((hydroxyimino)methyl)thiazol-2-yl)piperidine-1-carboxylate (4 g, 12.8 mmol) in ethyl acetate (500 mL) To the stirred solution was added N-chlorosuccinimide (2.57 g, 19.27 mmol) followed by sodium bicarbonate (7.55 g, 90 mmol). To the reaction mixture was added 3-chloro-2-vinylphenylmethanesulfonate (4.48 g, 19.27 mmol) and water (10 mL). The reaction mixture was heated to 65°C for 3 hours, cooled to 15°C and then quenched with water. The aqueous layer was extracted twice with ethyl acetate (50 mL). The combined ethyl acetate layers were dried over anhydrous sodium sulfate, concentrated a...
example 3
[0349] Example 3: Preparation of 2-((3-(trifluoromethyl)pyrazin-2-yl)oxy)acetic acid
[0350] Step A: Preparation of 3-(trifluoromethyl)pyrazin-2-ol
[0351] To a solution of pyrazin-2-ol (5 g, 52.0 mmol) and sodium trifluoromethanesulfinate (20.3 g, 130 mmol) in acetic acid (100 mL) was added manganese acetate dihydrate (41.9 g, 156 mmol) portionwise. The mixture was stirred at 25°C for 12 hours. Water (20 mL) was added to the reaction mixture and extracted twice with ethyl acetate. The combined ethyl acetate layers were dried over anhydrous sodium sulfate, concentrated and purified by column chromatography (using 70% ethyl acetate in hexanes as eluent) to give 3-(trifluoromethyl)pyrazine-2 - Alcohol (2.2 g, 13.41 mmol, 26% yield).
[0352] MS: m / z=165.10 (M+1)
[0353] Step B: Preparation of tert-butyl 2-((3-(trifluoromethyl)pyrazin-2-yl)oxy)acetate
[0354]To a solution of 3-(trifluoromethyl)pyrazin-2-ol (0.9 g, 5.48 mmol) in toluene (50 mL) at 25 °C was added silver c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com